Development of a new dipstick (Cholkit) for rapid detection of Vibrio cholerae O1 in acute watery diarrheal stools
- PMID: 29538377
- PMCID: PMC5862499
- DOI: 10.1371/journal.pntd.0006286
Development of a new dipstick (Cholkit) for rapid detection of Vibrio cholerae O1 in acute watery diarrheal stools
Abstract
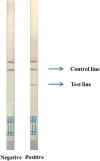
Recognizing cholera cases early, especially in the initial phase of an outbreak and in areas where cholera has not previously circulated, is a high public health priority. Laboratory capacity in such settings is often limited. To address this, we have developed a rapid diagnostic test (RDT) termed Cholkit that is based on an immunochromatographic lateral flow assay for the diagnosis of cholera cases using stool. Cholkit contains a monoclonal antibody (ICL-33) to the O-specific polysaccharide (OSP) component of V. cholerae O1 lipopolysaccharide, and recognizes both Inaba and Ogawa serotypes. We tested the Cholkit dipstick using fresh stool specimens of 76 adults and children presenting with acute watery diarrhea at the icddr,b hospital in Dhaka, Bangladesh. We compared Cholkit's performance with those of microbial culture, PCR (targeting the rfb and ctxA genes of V. cholerae) and the commercially available RDT, Crystal VC (Span Diagnostics; Surat, India). We found that all stool specimens with a positive culture for V. cholerae O1 (n = 19) were positive by Cholkit as well as Crystal VC. We then used Bayesian latent class modeling to estimate the sensitivity and specificity of each diagnostic assay. The sensitivity of Cholkit, microbiological culture, PCR and Crystal VC was 98% (95% CI: 88-100), 71% (95% CI: 59-81), 74% (95% CI: 59-86) and 98% (95% CI: 88-100), respectively. The specificity for V. cholerae O1 was 97% (95% CI: 89-100), 100%, 97% (95% CI: 93-99) and 98% (95% CI: 92-100), respectively. Of note, two Crystal VC dipsticks were positive for V. cholerae O139 but negative by culture and PCR in this area without known circulating epidemic V. cholerae O139. In conclusion, the Cholkit dipstick is simple to use, requires no dedicated laboratory capacity, and has a sensitivity and specificity for V. cholerae O1 of 98% and 97%, respectively. Cholkit warrants further evaluation in other settings.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: NJA, MNA, NS,and JA are working in a commercial company, Incepta Pharmaceuticals Ltd. These personnel are paid from Incepta Pharmaceuticals Ltd. They are working in collaboration with icddr,b to develop the product Cholkit.
Figures

Similar articles
-
Field evaluation of a locally produced rapid diagnostic test for early detection of cholera in Bangladesh.PLoS Negl Trop Dis. 2019 Jan 31;13(1):e0007124. doi: 10.1371/journal.pntd.0007124. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30703097 Free PMC article.
-
Laboratory evaluation of the rapid diagnostic tests for the detection of Vibrio cholerae O1 using diarrheal samples.PLoS Negl Trop Dis. 2021 Jun 15;15(6):e0009521. doi: 10.1371/journal.pntd.0009521. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34129602 Free PMC article.
-
Diagnostic limitations to accurate diagnosis of cholera.J Clin Microbiol. 2010 Nov;48(11):3918-22. doi: 10.1128/JCM.00616-10. Epub 2010 Aug 25. J Clin Microbiol. 2010. PMID: 20739485 Free PMC article.
-
Cholera Rapid Diagnostic Tests for the Detection of Vibrio cholerae O1: An Updated Meta-Analysis.Diagnostics (Basel). 2021 Nov 13;11(11):2095. doi: 10.3390/diagnostics11112095. Diagnostics (Basel). 2021. PMID: 34829444 Free PMC article. Review.
-
Diagnosis of Vibrio cholerae O1 infection in Africa.J Infect Dis. 2013 Nov 1;208 Suppl 1:S23-31. doi: 10.1093/infdis/jit196. J Infect Dis. 2013. PMID: 24101641 Review.
Cited by
-
Assembly and performance of a cholera RDT prototype that detects both Vibrio cholerae and associated bacteriophage as a proxy for pathogen detection.J Clin Microbiol. 2025 Feb 19;63(2):e0144324. doi: 10.1128/jcm.01443-24. Epub 2024 Dec 31. J Clin Microbiol. 2025. PMID: 39745446 Free PMC article.
-
Dynamics of IgM and IgA Antibody Response Profile Against Vibrio cholerae Toxins A, B, and P.Int J Mol Sci. 2025 Apr 9;26(8):3507. doi: 10.3390/ijms26083507. Int J Mol Sci. 2025. PMID: 40331989 Free PMC article.
-
Field Evaluation of Cholkit Rapid Diagnostic Test for Vibrio Cholerae O1 During a Cholera Outbreak in Malawi, 2018.Open Forum Infect Dis. 2020 Oct 16;7(11):ofaa493. doi: 10.1093/ofid/ofaa493. eCollection 2020 Nov. Open Forum Infect Dis. 2020. PMID: 33241067 Free PMC article.
-
Cholera rages in Africa and the Middle East: A narrative review on challenges and solutions.Health Sci Rep. 2024 May 12;7(5):e2013. doi: 10.1002/hsr2.2013. eCollection 2024 May. Health Sci Rep. 2024. PMID: 38742091 Free PMC article. Review.
-
Clinical Cholera Surveillance Sensitivity in Bangladesh and Implications for Large-Scale Disease Control.J Infect Dis. 2021 Dec 20;224(12 Suppl 2):S725-S731. doi: 10.1093/infdis/jiab418. J Infect Dis. 2021. PMID: 34453539 Free PMC article.
References
-
- Khazaei HA, Rezaei N, Bagheri GR, Moin AA. A six-year study on Vibrio cholerae in southeastern Iran. Japanese journal of infectious diseases. 2005;58(1):8–10. . - PubMed
-
- Keddy KH, Sooka A, Parsons MB, Njanpop-Lafourcade BM, Fitchet K, Smith AM. Diagnosis of Vibrio cholerae O1 infection in Africa. The Journal of infectious diseases. 2013;208 Suppl 1:S23–31. doi: 10.1093/infdis/jit196 . - DOI - PubMed
-
- Shears P. Recent developments in cholera. Current opinion in infectious diseases. 2001;14(5):553–8. . - PubMed
-
- Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85(13):117–28. Epub 2010/03/31. . - PubMed
-
- Organization WH. Prevention and control of cholera outbreaks: WHO policy and recommendations. Geneva: World Health Organization, Global Task Force on Cholera Control; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

