Modifying and reacting to the environmental pH can drive bacterial interactions
- PMID: 29538378
- PMCID: PMC5868856
- DOI: 10.1371/journal.pbio.2004248
Modifying and reacting to the environmental pH can drive bacterial interactions
Abstract
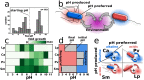
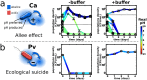
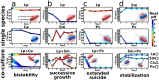
Microbes usually exist in communities consisting of myriad different but interacting species. These interactions are typically mediated through environmental modifications; microbes change the environment by taking up resources and excreting metabolites, which affects the growth of both themselves and also other microbes. We show here that the way microbes modify their environment and react to it sets the interactions within single-species populations and also between different species. A very common environmental modification is a change of the environmental pH. We find experimentally that these pH changes create feedback loops that can determine the fate of bacterial populations; they can either facilitate or inhibit growth, and in extreme cases will cause extinction of the bacterial population. Understanding how single species change the pH and react to these changes allowed us to estimate their pairwise interaction outcomes. Those interactions lead to a set of generic interaction motifs-bistability, successive growth, extended suicide, and stabilization-that may be independent of which environmental parameter is modified and thus may reoccur in different microbial systems.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Atlas RM, Bartha R. Microbial ecology: Fundamentals and applications. 1986. [Cited 16 February 2017]. https://www.osti.gov/scitech/biblio/5758135.
-
- Faust K, Raes J. Microbial interactions: from networks to models. Nat Rev Microbiol. 2012;10: 538–550. doi: 10.1038/nrmicro2832 - DOI - PubMed
-
- Fuhrman JA. Microbial community structure and its functional implications. Nature. 2009;459: 193–199. doi: 10.1038/nature08058 - DOI - PubMed
-
- Raes J, Bork P. Molecular eco-systems biology: towards an understanding of community function. Nat Rev Microbiol. 2008;6: 693–699. doi: 10.1038/nrmicro1935 - DOI - PubMed
-
- Strom SL. Microbial Ecology of Ocean Biogeochemistry: A Community Perspective. Science. 2008;320: 1043–1045. doi: 10.1126/science.1153527 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

