Functional autoantibodies in patients with different forms of dementia
- PMID: 29538413
- PMCID: PMC5851545
- DOI: 10.1371/journal.pone.0192778
Functional autoantibodies in patients with different forms of dementia
Erratum in
-
Correction: Functional autoantibodies in patients with different forms of dementia.PLoS One. 2018 Aug 27;13(8):e0203253. doi: 10.1371/journal.pone.0203253. eCollection 2018. PLoS One. 2018. PMID: 30148896 Free PMC article.
Abstract
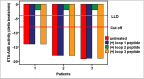
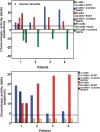
Dementia in general and Alzheimer's disease in particular is increasingly seen in association with autoimmunity being causatively or supportively involved in the pathogenesis. Besides classic autoantibodies (AABs) present in dementia patients, there is the new autoantibody class called functional autoantibodies, which is directed against G-protein coupled receptors (GPCRs; GPCR-AABs) and are seen as pathogenic players. However, less is known about dementia patients' burden with functional autoantibodies. We present here for the first time a study analyzing the prevalence of GPCR-AABs in patients with different dementia forms such as unclassified, Lewy body, vascular and Alzheimer's dementia. We identified the GPCR-AABs' specific targets on the receptors and introduced a neutralization strategy for GPCR-AABs. Patients with Alzheimer’s and vascular dementia carried GPCR-AABs targeting the second loop of the alpha1- and the first loop of the beta2-adrenergic receptors (α1-AABs; β2-AABs). The majority of patients with Lewy body dementia lacked any of the GPCR-AABs. In vitro, the function of the dementia-associated GPCR-AABs could be neutralized by the aptamer BC007. Due to the presence of GPCR-AABs in dementia patients mainly in those suffering from Alzheimer's and vascular dementia, the orchestra of immune players in these dementia forms, so far preferentially represented by the classic autoantibodies, should be supplemented by functional autoantibodies. As dementia-associated functional autoantibodies could be neutralized by the aptamer BC007, the first step was taken for a new in vivo treatment option in dementia patients who were positive for GPCR-AABs.
Conflict of interest statement
Figures




Similar articles
-
Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: A vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies.Atherosclerosis. 2016 Jan;244:44-7. doi: 10.1016/j.atherosclerosis.2015.11.001. Epub 2015 Nov 10. Atherosclerosis. 2016. PMID: 26584137
-
Autoantibodies Directed Against the Endothelin A Receptor in Patients With Benign Prostatic Hyperplasia.Prostate. 2017 Apr;77(5):458-465. doi: 10.1002/pros.23284. Epub 2016 Nov 24. Prostate. 2017. PMID: 27882567
-
Cardiomyopathy - An approach to the autoimmune background.Autoimmun Rev. 2017 Mar;16(3):269-286. doi: 10.1016/j.autrev.2017.01.012. Epub 2017 Feb 2. Autoimmun Rev. 2017. PMID: 28163240 Review.
-
Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases.Semin Immunopathol. 2014 May;36(3):351-63. doi: 10.1007/s00281-014-0425-9. Epub 2014 Apr 29. Semin Immunopathol. 2014. PMID: 24777744 Review.
-
Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies.Circ Res. 2011 Oct 14;109(9):986-92. doi: 10.1161/CIRCRESAHA.111.253849. Epub 2011 Aug 25. Circ Res. 2011. PMID: 21868696
Cited by
-
The role of autoantibodies in Alzheimer's disease: Pathogenetic connections or epiphenomena?Alzheimers Dement. 2025 Jul;21(7):e70484. doi: 10.1002/alz.70484. Alzheimers Dement. 2025. PMID: 40696840 Free PMC article. Review.
-
Glaucoma and Alzheimer: Neurodegenerative disorders show an adrenergic dysbalance.PLoS One. 2022 Oct 6;17(10):e0272811. doi: 10.1371/journal.pone.0272811. eCollection 2022. PLoS One. 2022. PMID: 36201426 Free PMC article.
-
Pathogenic roles of autoantibodies in systemic sclerosis: Current understandings in pathogenesis.J Scleroderma Relat Disord. 2020 Jun;5(2):103-129. doi: 10.1177/2397198319870667. Epub 2019 Sep 9. J Scleroderma Relat Disord. 2020. PMID: 35382028 Free PMC article. Review.
-
Autoimmune encephalitis in the elderly: who to test and what to test for.Evid Based Ment Health. 2019 Nov;22(4):172-176. doi: 10.1136/ebmental-2019-300110. Epub 2019 Sep 19. Evid Based Ment Health. 2019. PMID: 31537612 Free PMC article. Review.
-
Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms.J Transl Autoimmun. 2021;4:100100. doi: 10.1016/j.jtauto.2021.100100. Epub 2021 Apr 16. J Transl Autoimmun. 2021. PMID: 33880442 Free PMC article.
References
-
- Colasanti T, Barbati C, Rosano G, Malorni W, Ortona E (2010) Autoantibodies in patients with Alzheimer's disease: pathogenetic role and potential use as biomarkers of disease progression. Autoimmun Rev 9: 807–811. Erratum in: Autoimmun Rev 11:374. doi: 10.1016/j.autrev.2010.07.008 - DOI - PubMed
-
- Wu J, Li L (2016) Autoantibodies in Alzheimer's disease: potential biomarkers, pathogenic roles, and therapeutic implications. J Biomed Res 30: 361–372. doi: 10.7555/JBR.30.20150131 - DOI - PMC - PubMed
-
- Wallukat G, Schimke I (2014) Agonistic autoantibodies directed against G-protein-coupled receptors and their relationship to cardiovascular diseases. Semin Immunopathol 36: 351–363. doi: 10.1007/s00281-014-0425-9 - DOI - PubMed
-
- Bornholz B, Wallukat G, Roggenbuck D, Schimke I. Autoantibodies against G-protein-coupled receptors in cardiovascular diseases: basics and diagnostics In: Nussinovitch U, editor. The heart in rheumatologic, inflammatory and autoimmune diseases: pathophysiology, clinical aspects and therapeutic approaches. Amsterdam: Elsevier; 2017. pp 49–63. eBook ISBN: 9780128032688; Hardcover ISBN: 9780128032671
-
- Müller J, Wallukat G, Schimke I. Autoantibody-directed therapy in cardiovascular diseases In: Nussinovitch U, editor. The heart in rheumatologic, inflammatory and autoimmune diseases: pathophysiology, clinical aspects and therapeutic approaches. Amsterdam: Elsevier, 2017. pp 659–679. eBook ISBN: 9780128032688; Hardcover ISBN: 9780128032671
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

