Tis21-gene therapy inhibits medulloblastoma growth in a murine allograft model
- PMID: 29538458
- PMCID: PMC5851620
- DOI: 10.1371/journal.pone.0194206
Tis21-gene therapy inhibits medulloblastoma growth in a murine allograft model
Abstract
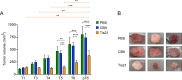
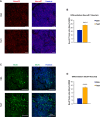
Medulloblastoma (MB), the tumor of the cerebellum, is the most frequent brain cancer in childhood and a major cause of pediatric mortality. Based on gene profiling, four MB subgroups have been identified, i.e., Wnt or Sonic Hedgehog (Shh) types, and subgroup 3 or 4. The Shh-type MB has been shown to arise from the cerebellar precursors of granule neurons (GCPs), where a hyperactivation of the Shh pathway leads to their neoplastic transformation. We have previously shown that the gene Tis21 (PC3/Btg2) inhibits the proliferation and promotes the differentiation and migration of GCPs. Moreover, the overexpression or the deletion of Tis21 in Patched1 heterozygous mice, a model of spontaneous Shh-type MB, highly reduces or increases, respectively, the frequency of MB. Here we tested whether Tis21 can inhibit MB allografts. Athymic nude mice were subcutaneously grafted with MB cells explanted from Patched1 heterozygous mice. MB allografts were then injected with adeno-associated viruses either carrying Tis21 (AAV-Tis21) or empty (AAV-CBA). We observed that the treatment with AAV-Tis21 significantly inhibited the growth of tumor nodules, as judged by their volume, and reduced the number of proliferating tumor cells (labeled with Ki67 or BrdU), relative to AAV-CBA-treated control mice. In parallel, AAV-Tis21 increased significantly tumor cells labeled with early and late neural differentiation markers. Overall the results suggest that Tis21-gene therapy slows down MB tumor growth through inhibition of proliferation and enhancement of neural differentiation. These results validate Tis21 as a relevant target for MB therapy.
Conflict of interest statement
Figures



References
-
- Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19: 1541–1544. doi: 10.1016/j.jocn.2012.04.009 - DOI - PubMed
-
- Brandão LA, Young Poussaint T. Posterior Fossa Tumors. Neuroimaging Clin N Am. 2017;27: 1–37. doi: 10.1016/j.nic.2016.08.001 - DOI - PubMed
-
- Rodini CO, Suzuki DE, Nakahata AM, Pereira MC, Janjoppi L, Toledo SR, et al. Aberrant signaling pathways in medulloblastomas: a stem cell connection. Arq Neuropsiquiatr. 2010;68: 947–952. - PubMed
-
- Crawford JR, MacDonald TJ, Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6: 1073–1085. doi: 10.1016/S1474-4422(07)70289-2 - DOI - PubMed
-
- Massimino M, Biassoni V, Gandola L, Garrè ML, Gatta G, Giangaspero F, et al. Childhood medulloblastoma. Crit Rev Oncol Hematol. 2016;105: 35–51. doi: 10.1016/j.critrevonc.2016.05.012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

