Acetaminophen as a Renoprotective Adjunctive Treatment in Patients With Severe and Moderately Severe Falciparum Malaria: A Randomized, Controlled, Open-Label Trial
- PMID: 29538635
- PMCID: PMC6137116
- DOI: 10.1093/cid/ciy213
Acetaminophen as a Renoprotective Adjunctive Treatment in Patients With Severe and Moderately Severe Falciparum Malaria: A Randomized, Controlled, Open-Label Trial
Abstract
Background: Acute kidney injury independently predicts mortality in falciparum malaria. It is unknown whether acetaminophen's capacity to inhibit plasma hemoglobin-mediated oxidation is renoprotective in severe malaria.
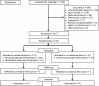
Methods: This phase 2, open-label, randomized controlled trial conducted at two hospitals in Bangladesh assessed effects on renal function, safety, pharmacokinetic (PK) properties and pharmacodynamic (PD) effects of acetaminophen. Febrile patients (>12 years) with severe falciparum malaria were randomly assigned to receive acetaminophen (1 g 6-hourly for 72 hours) or no acetaminophen, in addition to intravenous artesunate. Primary outcome was the proportional change in creatinine after 72 hours stratified by median plasma hemoglobin.
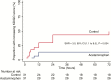
Results: Between 2012 and 2014, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31). Median (interquartile range) reduction in creatinine after 72 hours was 23% (37% to 18%) in patients assigned to acetaminophen, versus 14% (29% to 0%) in patients assigned to no acetaminophen (P = .043). This difference in reduction was 37% (48% to 22%) versus 14% (30% to -71%) in patients with hemoglobin ≥45000 ng/mL (P = .010). The proportion with progressing kidney injury was higher among controls (subdistribution hazard ratio, 3.0; 95% confidence interval, 1.1 to 8.5; P = .034). PK-PD analyses showed that higher exposure to acetaminophen increased the probability of creatinine improvement. No patient fulfilled Hy's law for hepatotoxicity.
Conclusions: In this proof-of-principle study, acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, particularly in those with prominent intravascular hemolysis.
Clinical trials registration: NCT01641289.
Figures

References
-
- Dondorp A, Nosten F, Stepniewska K, Day N, White N; South East Asian Quinine Artesunate Malaria Trial Group Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717–25. - PubMed
-
- Trang TT, Phu NH, Vinh H, et al. . Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis 1992; 15:874–80. - PubMed
-
- Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, et al. . Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int 2010; 77:913–20. - PubMed
-
- Nair RK, Khaira A, Sharma A, Mahajan S, Dinda AK. Spectrum of renal involvement in paroxysmal nocturnal hemoglobinuria: report of three cases and a brief review of the literature. Int Urol Nephrol 2008; 40:471–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

