Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection in Patients at Increased Risk for Recurrence
- PMID: 29538686
- PMCID: PMC6093994
- DOI: 10.1093/cid/ciy171
Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection in Patients at Increased Risk for Recurrence
Abstract
Background: Bezlotoxumab is a human monoclonal antibody against Clostridium difficile toxin B indicated to prevent C. difficile infection (CDI) recurrence (rCDI) in adults at high risk for rCDI. This post hoc analysis of pooled monocolonal antibodies for C.difficile therapy (MODIFY) I/II data assessed bezlotoxumab efficacy in participants with characteristics associated with increased risk for rCDI.
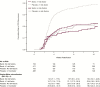
Methods: The analysis population was the modified intent-to-treat population who received bezlotoxumab or placebo (n = 1554) by risk factors for rCDI that were prespecified in the statistical analysis plan: age ≥65 years, history of CDI, compromised immunity, severe CDI, and ribotype 027/078/244. The proportion of participants with rCDI in 12 weeks, fecal microbiota transplant procedures, 30-day all cause and CDI-associated hospital readmissions, and mortality at 30 and 90 days after randomization were presented.
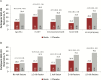
Results: The majority of enrolled participants (75.6%) had ≥1 risk factor; these participants were older and a higher proportion had comorbidities compared with participants with no risk factors. The proportion of placebo participants who experienced rCDI exceeded 30% for each risk factor compared with 20.9% among those without a risk factor, and the rCDI rate increased with the number of risk factors (1 risk factor: 31.3%; ≥3 risk factors: 46.1%). Bezlotoxumab reduced rCDI, fecal microbiota transplants, and CDI-associated 30-day readmissions in participants with risk factors for rCDI.
Conclusions: The risk factors prespecified in the MODIFY statistical analysis plan are appropriate to identify patients at high risk for rCDI. While participants with ≥3 risk factors had the greatest reduction of rCDI with bezlotoxumab, those with 1 or 2 risk factors may also benefit.
Clinical trials registration: NCT01241552 (MODIFY I) and NCT01513239 (MODIFY II).
Figures

References
-
- Lagier J. Gut microbiota and Clostridium difficile infections. Hum Microbiome J 2016; 2:10–4.
-
- Louie TJ, Miller MA, Mullane KM, et al. ; OPT-80-003 Clinical Study Group Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–31. - PubMed
-
- Johnson S, Louie TJ, Gerding DN, et al. ; Polymer Alternative for CDI Treatment (PACT) Investigators Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. - PubMed
-
- Sheitoyan-Pesant C, Abou Chakra CN, Pépin J, Marcil-Héguy A, Nault V, Valiquette L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis 2016; 62:574–80. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

