Placentation defects are highly prevalent in embryonic lethal mouse mutants
- PMID: 29539633
- PMCID: PMC5866719
- DOI: 10.1038/nature26002
Placentation defects are highly prevalent in embryonic lethal mouse mutants
Abstract
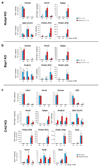
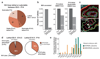
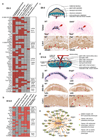
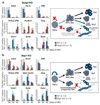
Large-scale phenotyping efforts have demonstrated that approximately 25-30% of mouse gene knockouts cause intrauterine lethality. Analysis of these mutants has largely focused on the embryo and not the placenta, despite the crucial role of this extraembryonic organ for developmental progression. Here we screened 103 embryonic lethal and sub-viable mouse knockout lines from the Deciphering the Mechanisms of Developmental Disorders program for placental phenotypes. We found that 68% of knockout lines that are lethal at or after mid-gestation exhibited placental dysmorphologies. Early lethality (embryonic days 9.5-14.5) is almost always associated with severe placental malformations. Placental defects correlate strongly with abnormal brain, heart and vascular development. Analysis of mutant trophoblast stem cells and conditional knockouts suggests that a considerable number of factors that cause embryonic lethality when ablated have primary gene function in trophoblast cells. Our data highlight the hugely under-appreciated importance of placental defects in contributing to abnormal embryo development and suggest key molecular nodes that govern placenta formation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures











References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

