Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells
- PMID: 29539635
- PMCID: PMC6054302
- DOI: 10.1038/nature25976
Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells
Erratum in
-
Author Correction: Diffusible repression of cytokinin signalling produces endodermal symmetry and passage cells.Nature. 2018 Jul;559(7714):E9. doi: 10.1038/s41586-018-0231-y. Nature. 2018. PMID: 29925940
Abstract
In vascular plants, the root endodermis surrounds the central vasculature as a protective sheath that is analogous to the polarized epithelium in animals, and contains ring-shaped Casparian strips that restrict diffusion. After an initial lag phase, individual endodermal cells suberize in an apparently random fashion to produce 'patchy' suberization that eventually generates a zone of continuous suberin deposition. Casparian strips and suberin lamellae affect paracellular and transcellular transport, respectively. Most angiosperms maintain some isolated cells in an unsuberized state as so-called 'passage cells', which have previously been suggested to enable uptake across an otherwise-impermeable endodermal barrier. Here we demonstrate that these passage cells are late emanations of a meristematic patterning process that reads out the underlying non-radial symmetry of the vasculature. This process is mediated by the non-cell-autonomous repression of cytokinin signalling in the root meristem, and leads to distinct phloem- and xylem-pole-associated endodermal cells. The latter cells can resist abscisic acid-dependent suberization to produce passage cells. Our data further demonstrate that, during meristematic patterning, xylem-pole-associated endodermal cells can dynamically alter passage-cell numbers in response to nutrient status, and that passage cells express transporters and locally affect the expression of transporters in adjacent cortical cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



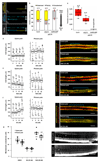
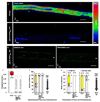
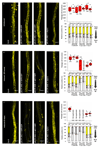
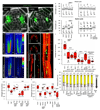


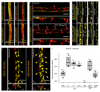

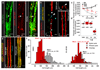



Comment in
-
A cellular passage to the root interior.Nature. 2018 Mar 22;555(7697):454-455. doi: 10.1038/d41586-018-02861-x. Nature. 2018. PMID: 29565372 No abstract available.
References
-
- Geldner N. The Endodermis. Annu Rev Plant Biol. 2013;64:531–558. - PubMed
-
- Barberon M, et al. Adaptation of Root Function by Nutrient-Induced Plasticity of Endodermal Differentiation. Cell. 2016;164:447–459. - PubMed
-
- Peterson CA, Enstone DE. Functions of passage cells in the endodermis and exodermis of roots. Physiol Plant. 1996;97:592–598.
-
- Kroemer H. Hypodermis und Endodermis der Angiospermenwurzel. 1903 Available at: https://www.schweizerbart.de/publications/detail/artno/144005900/Bibliot....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

