Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk
- PMID: 29540217
- PMCID: PMC5870815
- DOI: 10.1186/s12933-018-0682-3
Rationale, design, and baseline characteristics of the CArdiovascular safety and Renal Microvascular outcomE study with LINAgliptin (CARMELINA®): a randomized, double-blind, placebo-controlled clinical trial in patients with type 2 diabetes and high cardio-renal risk
Abstract
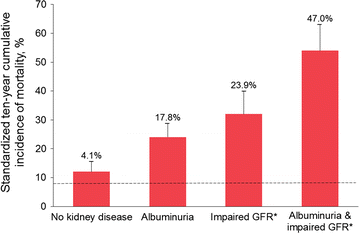
Background: Cardiovascular (CV) outcome trials in type 2 diabetes (T2D) have underrepresented patients with chronic kidney disease (CKD), leading to uncertainty regarding their kidney efficacy and safety. The CARMELINA® trial aims to evaluate the effects of linagliptin, a DPP-4 inhibitor, on both CV and kidney outcomes in a study population enriched for cardio-renal risk.
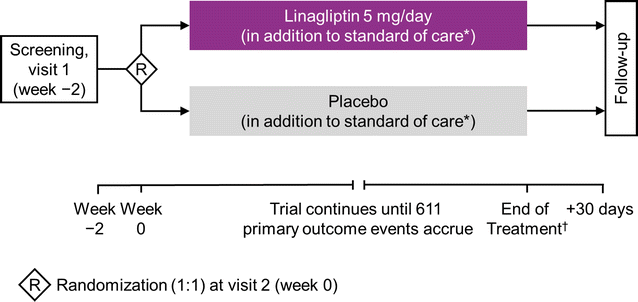
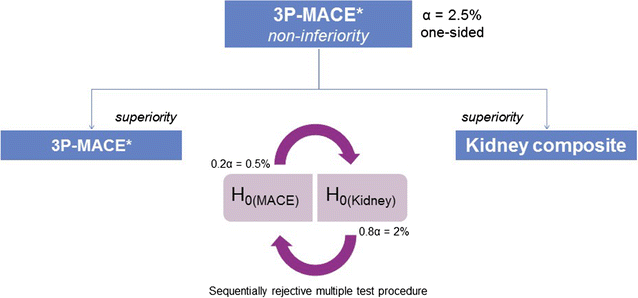
Methods: CARMELINA® is a randomized, double-blind, placebo-controlled clinical trial conducted in 27 countries in T2D patients at high risk of CV and/or kidney events. Participants with evidence of CKD with or without CV disease and HbA1c 6.5-10.0% (48-86 mmol/mol) were randomized 1:1 to receive linagliptin once daily or matching placebo, added to standard of care adjusted according to local guidelines. The primary outcome is time to first occurrence of CV death, non-fatal myocardial infarction, or non-fatal stroke. The key secondary outcome is a composite of time to first sustained occurrence of end-stage kidney disease, ≥ 40% decrease in estimated glomerular filtration rate (eGFR) from baseline, or renal death. CV and kidney events are prospectively adjudicated by independent, blinded clinical event committees. CARMELINA® was designed to continue until at least 611 participants had confirmed primary outcome events. Assuming a hazard ratio of 1.0, this provides 90% power to demonstrate non-inferiority of linagliptin versus placebo within the pre-specified non-inferiority margin of 1.3 at a one-sided α-level of 2.5%. If non-inferiority of linagliptin for the primary outcome is demonstrated, then its superiority for both the primary outcome and the key secondary outcome will be investigated with a sequentially rejective multiple test procedure.
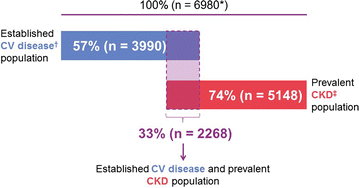
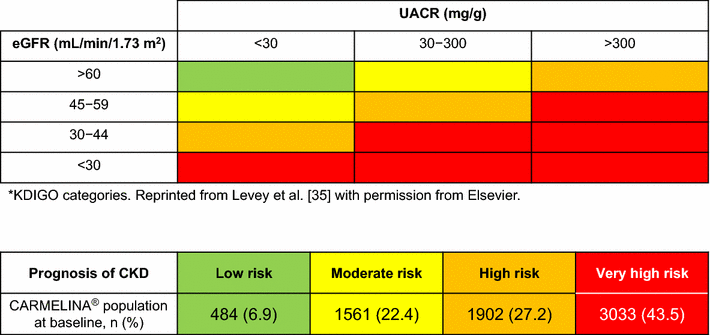
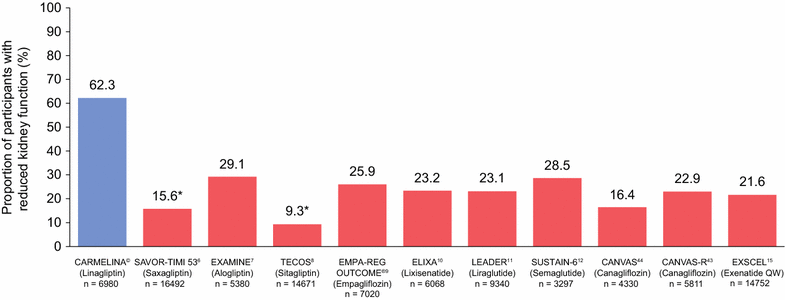
Results: Between July 2013 and August 2016, 6980 patients were randomized and took ≥ 1 dose of study drug (40.6, 33.1, 16.9, and 9.4% from Europe, South America, North America, and Asia, respectively). At baseline, mean ± SD age was 65.8 ± 9.1 years, HbA1c 7.9 ± 1.0%, BMI 31.3 ± 5.3 kg/m2, and eGFR 55 ± 25 mL/min/1.73 m2. A total of 5148 patients (73.8%) had prevalent kidney disease (defined as eGFR < 60 mL/min/1.73 m2 or macroalbuminuria [albumin-to-creatinine ratio > 300 mg/g]) and 3990 patients (57.2%) had established CV disease with increased albuminuria; these characteristics were not mutually exclusive. Microalbuminuria (n = 2896 [41.5%]) and macroalbuminuria (n = 2691 [38.6%]) were common.
Conclusions: CARMELINA® will add important information regarding the CV and kidney disease clinical profile of linagliptin by including an understudied, vulnerable cohort of patients with T2D at highest cardio-renal risk. Trial registration ClinicalTrials.gov identifier-NCT01897532; registered July 9, 2013.
Keywords: Cardiovascular diseases; Clinical trial, phase IV; Diabetes mellitus, type 2; Diabetic nephropathies; Dipeptidyl-peptidase IV inhibitors; Linagliptin; Research design; Treatment outcome.
Figures
Similar articles
-
Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial.JAMA. 2019 Jan 1;321(1):69-79. doi: 10.1001/jama.2018.18269. JAMA. 2019. PMID: 30418475 Free PMC article. Clinical Trial.
-
Effects of Linagliptin on Cardiovascular and Kidney Outcomes in People With Normal and Reduced Kidney Function: Secondary Analysis of the CARMELINA Randomized Trial.Diabetes Care. 2020 Aug;43(8):1803-1812. doi: 10.2337/dc20-0279. Epub 2020 May 22. Diabetes Care. 2020. PMID: 32444457 Free PMC article. Clinical Trial.
-
Linagliptin Effects on Heart Failure and Related Outcomes in Individuals With Type 2 Diabetes Mellitus at High Cardiovascular and Renal Risk in CARMELINA.Circulation. 2019 Jan 15;139(3):351-361. doi: 10.1161/CIRCULATIONAHA.118.038352. Circulation. 2019. PMID: 30586723 Clinical Trial.
-
Dipeptidyl peptidase-4 inhibitors and cardiovascular and renal disease in type 2 diabetes: What have we learned from the CARMELINA trial?Diab Vasc Dis Res. 2019 Jul;16(4):303-309. doi: 10.1177/1479164119842339. Epub 2019 Apr 24. Diab Vasc Dis Res. 2019. PMID: 31018682 Free PMC article. Review.
-
Class effects of SGLT2 inhibitors on cardiorenal outcomes.Cardiovasc Diabetol. 2019 Aug 5;18(1):99. doi: 10.1186/s12933-019-0903-4. Cardiovasc Diabetol. 2019. PMID: 31382965 Free PMC article. Review.
Cited by
-
Platelet Effects of Anti-diabetic Therapies: New Perspectives in the Management of Patients with Diabetes and Cardiovascular Disease.Front Pharmacol. 2021 May 12;12:670155. doi: 10.3389/fphar.2021.670155. eCollection 2021. Front Pharmacol. 2021. PMID: 34054542 Free PMC article. Review.
-
Renal Outcomes of Antidiabetic Treatment Options for Type 2 Diabetes-A Proposed MARE Definition.Kidney Int Rep. 2018 Apr 22;3(5):1030-1038. doi: 10.1016/j.ekir.2018.04.008. eCollection 2018 Sep. Kidney Int Rep. 2018. PMID: 30197969 Free PMC article. Review.
-
Efficacy of anagliptin as compared to linagliptin on metabolic parameters over 2 years of drug consumption: A retrospective cohort study.World J Diabetes. 2018 Oct 15;9(10):165-171. doi: 10.4239/wjd.v9.i10.165. World J Diabetes. 2018. PMID: 30364744 Free PMC article.
-
Review of the cardiovascular safety of dipeptidyl peptidase-4 inhibitors and the clinical relevance of the CAROLINA trial.BMC Cardiovasc Disord. 2019 Mar 15;19(1):60. doi: 10.1186/s12872-019-1036-0. BMC Cardiovasc Disord. 2019. PMID: 30876392 Free PMC article. Review.
-
Should Metformin Remain First-Line Medical Therapy for Patients with Type 2 Diabetes Mellitus and Atherosclerotic Cardiovascular Disease? An Alternative Approach.Curr Diab Rep. 2018 Jul 14;18(9):64. doi: 10.1007/s11892-018-1035-z. Curr Diab Rep. 2018. PMID: 30008022 Review.
References
-
- Center for Drug Evaluation and Research. Guidance for industry. Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring, MD, U.S. Food and Drug Administration; 2008. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati.... Accessed 10 July 2017.
-
- Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. London. European Medicines Agency; 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 10 July 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

