The blood fluke Schistosoma mansoni cleaves the coagulation protein high molecular weight kininogen (HK) but does not generate the vasodilator bradykinin
- PMID: 29540224
- PMCID: PMC5853081
- DOI: 10.1186/s13071-018-2704-0
The blood fluke Schistosoma mansoni cleaves the coagulation protein high molecular weight kininogen (HK) but does not generate the vasodilator bradykinin
Abstract
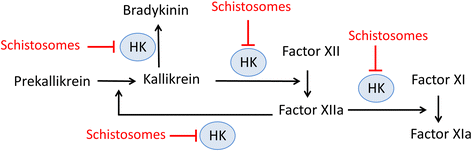
Background: Schistosomes are blood dwelling parasitic worms that cause the debilitating disease schistosomiasis. Here we examined the influence of the parasites on their external environment by monitoring the impact of adult Schistosoma mansoni worms on the murine plasma proteome in vitro and, in particular, on how the worms affect the blood coagulation protein high molecular weight kininogen (HK).
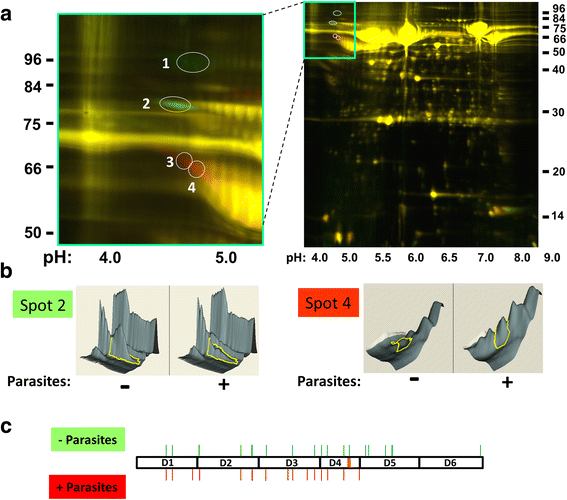
Methods: Following the incubation of adult schistosomes in murine plasma, two-dimensional differential in-gel electrophoresis (2D-DIGE) was conducted to look for changes in the plasma proteome compared with control plasma. A major change to the blood protein kininogen (HK) was observed, and the interaction of Schistosoma mansoni parasite with this protein alone was then investigated by western blot analysis and activity assays. Finally, the generation of bradykinin from HK was monitored using a bradykinin detection kit.
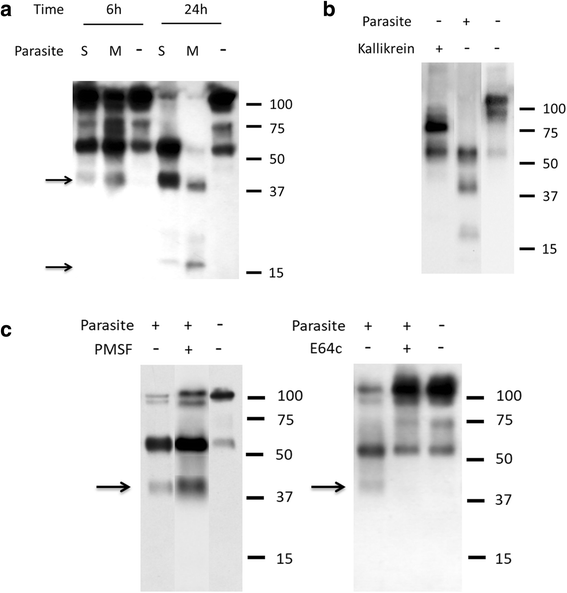
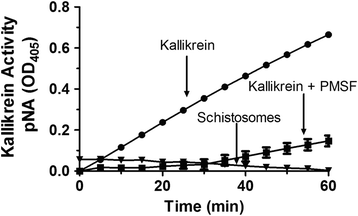
Results: The most striking change to the plasma proteome concerned HK; while the full-length protein was more abundant in control plasma, carboxyl-terminal truncated forms were more abundant in plasma that contained schistosomes. Incubating parasites in buffer with pure HK followed by Western blot analysis confirmed that human HK is degraded by the worms. The resulting digestion pattern differed from that brought about by kallikrein, a host serine protease that normally acts on HK to release the vasodilator bradykinin. We found that live schistosomes, while digesting HK, do not generate bradykinin nor do they cleave a chromogenic kallikrein substrate. Since the cleavage of HK by the worms is not impeded by the serine protease inhibitor PMSF but is blocked by the cysteine protease inhibitor E64c, we hypothesize that schistosome tegumental cysteine proteases are responsible for HK cleavage.
Conclusions: Since proteomic and biochemical studies have revealed that the schistosome tegument contains two cysteine proteases belonging to the calpain family (SmCalp1 and SmCalp2) we conclude that these are likely responsible for the HK cleavage reported here. Schistosome cleavage of HK should help impede blood clotting and inflammation around the worms in vivo and so promote their ease of movement within the vasculature of their hosts.
Keywords: Bradykinin; Calpain; Coagulation; High molecular weight kininogen; Schistosoma mansoni.
Conflict of interest statement
Ethics approval and consent to participate
All protocols involving animals were approved by the Institutional Animal Care and Use Committees (IACUC) of Tufts University under protocol G2015-113. The study did not involve human subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
The human blood parasite Schistosoma mansoni expresses extracellular tegumental calpains that cleave the blood clotting protein fibronectin.Sci Rep. 2017 Oct 10;7(1):12912. doi: 10.1038/s41598-017-13141-5. Sci Rep. 2017. PMID: 29018227 Free PMC article.
-
Factor XII-independent cleavage of high-molecular-weight kininogen by prekallikrein and inhibition by C1 inhibitor.J Allergy Clin Immunol. 2009 Jul;124(1):143-9. doi: 10.1016/j.jaci.2009.02.006. Epub 2009 Apr 1. J Allergy Clin Immunol. 2009. PMID: 19342086
-
The hyaluronan-binding serine protease from human plasma cleaves HMW and LMW kininogen and releases bradykinin.Biol Chem. 2002 Oct;383(10):1633-43. doi: 10.1515/BC.2002.184. Biol Chem. 2002. PMID: 12452440
-
Pathogenic mechanisms of bradykinin mediated diseases: dysregulation of an innate inflammatory pathway.Adv Immunol. 2014;121:41-89. doi: 10.1016/B978-0-12-800100-4.00002-7. Adv Immunol. 2014. PMID: 24388213 Review.
-
The bradykinin-forming cascade: a historical perspective.Chem Immunol Allergy. 2014;100:205-13. doi: 10.1159/000358739. Epub 2014 May 22. Chem Immunol Allergy. 2014. PMID: 24925400 Review.
Cited by
-
Schistosoma mansoni glyceraldehyde-3-phosphate dehydrogenase enhances formation of the blood-clot lysis protein plasmin.Biol Open. 2020 Mar 24;9(3):bio050385. doi: 10.1242/bio.050385. Biol Open. 2020. PMID: 32098782 Free PMC article.
-
Human serum activates the tegument of female schistosomes and supports recovery from Praziquantel.Parasitol Res. 2021 Jan;120(1):209-221. doi: 10.1007/s00436-020-06968-x. Epub 2020 Dec 1. Parasitol Res. 2021. PMID: 33263166 Free PMC article.
-
Identification and profiling of Trichinella spiralis circulating antigens and proteins in sera of mice with trichinellosis.PLoS One. 2022 Mar 10;17(3):e0265013. doi: 10.1371/journal.pone.0265013. eCollection 2022. PLoS One. 2022. PMID: 35271623 Free PMC article.
-
Kinins and Their Receptors in Infectious Diseases.Pharmaceuticals (Basel). 2020 Aug 27;13(9):215. doi: 10.3390/ph13090215. Pharmaceuticals (Basel). 2020. PMID: 32867272 Free PMC article. Review.
-
COVID-19 and Diarylamidines: The Parasitic Connection.Int J Mol Sci. 2023 Apr 1;24(7):6583. doi: 10.3390/ijms24076583. Int J Mol Sci. 2023. PMID: 37047556 Free PMC article. Review.
References
-
- World Health Organization. Schistosomiasis Fact Sheet. http://www.who.int/mediacentre/factsheets/fs115/en/. Accessed 1 Oct 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

