Rewiring the connectome: Evidence and effects
- PMID: 29540321
- PMCID: PMC5903872
- DOI: 10.1016/j.neubiorev.2018.03.001
Rewiring the connectome: Evidence and effects
Abstract
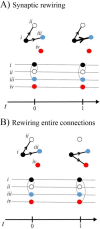
Neuronal connections form the physical basis for communication in the brain. Recently, there has been much interest in mapping the "connectome" to understand how brain structure gives rise to brain function, and ultimately, to behaviour. These attempts to map the connectome have largely assumed that connections are stable once formed. Recent studies, however, indicate that connections in mammalian brains may undergo rewiring during learning and experience-dependent plasticity. This suggests that the connectome is more dynamic than previously thought. To what extent can neural circuitry be rewired in the healthy adult brain? The connectome has been subdivided into multiple levels of scale, from synapses and microcircuits through to long-range tracts. Here, we examine the evidence for rewiring at each level. We then consider the role played by rewiring during learning. We conclude that harnessing rewiring offers new avenues to treat brain diseases.
Keywords: Axon; Behaviour; Cognition; Computational; Cortex; Dendrite; Learning; Memory; Network; Neuron; Neuropsychiatric; Stroke; Synapse; fMRI.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

Similar articles
-
A Dynamic Connectome Supports the Emergence of Stable Computational Function of Neural Circuits through Reward-Based Learning.eNeuro. 2018 Apr 24;5(2):ENEURO.0301-17.2018. doi: 10.1523/ENEURO.0301-17.2018. eCollection 2018 Mar-Apr. eNeuro. 2018. PMID: 29696150 Free PMC article.
-
Altered sensory experience induces targeted rewiring of local excitatory connections in mature neocortex.J Neurosci. 2008 Sep 10;28(37):9249-60. doi: 10.1523/JNEUROSCI.2974-08.2008. J Neurosci. 2008. PMID: 18784305 Free PMC article.
-
Synapse rearrangements upon learning: from divergent-sparse connectivity to dedicated sub-circuits.Trends Neurosci. 2014 Oct;37(10):604-14. doi: 10.1016/j.tins.2014.08.011. Epub 2014 Sep 22. Trends Neurosci. 2014. PMID: 25257207 Review.
-
[Neurobiology of learning--the basis of an alteration process].Psychiatr Prax. 2004 Nov;31 Suppl 2:S215-23. doi: 10.1055/s-2004-828485. Psychiatr Prax. 2004. PMID: 15586313 Review. German.
-
Sensory experience and cortical rewiring.Neuroscientist. 2010 Apr;16(2):186-98. doi: 10.1177/1073858409343961. Epub 2009 Oct 1. Neuroscientist. 2010. PMID: 19801372 Review.
Cited by
-
Effect of Acupuncture Stimulation of Hegu (LI4) and Taichong (LR3) on the Resting-State Networks in Alzheimer's Disease: Beyond the Default Mode Network.Neural Plast. 2021 Mar 8;2021:8876873. doi: 10.1155/2021/8876873. eCollection 2021. Neural Plast. 2021. PMID: 33747074 Free PMC article.
-
Perinatal Penicillin Exposure Affects Cortical Development and Sensory Processing.Front Mol Neurosci. 2021 Dec 22;14:704219. doi: 10.3389/fnmol.2021.704219. eCollection 2021. Front Mol Neurosci. 2021. PMID: 35002614 Free PMC article.
-
From Topological Analyses to Functional Modeling: The Case of Hippocampus.Front Comput Neurosci. 2021 Jan 11;14:593166. doi: 10.3389/fncom.2020.593166. eCollection 2020. Front Comput Neurosci. 2021. PMID: 33505262 Free PMC article.
-
The role of nuclear Ca2+ in maintaining neuronal homeostasis and brain health.J Cell Sci. 2021 Apr 15;134(8):jcs254904. doi: 10.1242/jcs.254904. Epub 2021 Apr 22. J Cell Sci. 2021. PMID: 33912918 Free PMC article. Review.
-
The impact of neuron morphology on cortical network architecture.Cell Rep. 2022 Apr 12;39(2):110677. doi: 10.1016/j.celrep.2022.110677. Cell Rep. 2022. PMID: 35417720 Free PMC article.
References
-
- Aertsen A.M., Gerstein G.L., Habib M.K., Palm G. Dynamics of neuronal firing correlation: modulation of "effective connectivity". J. Neurophysiol. 1989;61:900–917. - PubMed
-
- Akers K.G., Martinez-Canabal A., Restivo L., Yiu A.P., De Cristofaro A., Hsiang H.L., Wheeler A.L., Guskjolen A., Niibori Y., Shoji H., Ohira K., Richards B.A., Miyakawa T., Josselyn S.A., Frankland P.W. Hippocampal neurogenesis regulates forgetting during adulthood and infancy. Science. 2014;344:598–602. - PubMed
-
- Anderson J.S., Nielsen J.A., Froehlich A.L., DuBray M.B., Druzgal T.J., Cariello A.N., Cooperrider J.R., Zielinski B.A., Ravichandran C., Fletcher P.T., Alexander A.L., Bigler E.D., Lange N., Lainhart J.E. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134:3742–3754. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

