Randomised controlled trial protocol to evaluate a fixed dose prothrombin complex concentrate against the variable dose in vitamin K antagonist related bleeding (PROPER3)
- PMID: 29540424
- PMCID: PMC5857685
- DOI: 10.1136/bmjopen-2017-020764
Randomised controlled trial protocol to evaluate a fixed dose prothrombin complex concentrate against the variable dose in vitamin K antagonist related bleeding (PROPER3)
Abstract
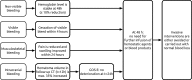
Introduction: There is currently little evidence for the optimal dosing strategy of four-factor prothrombin complex concentrates (PCC) in vitamin K antagonist (VKA)-related bleeds. The generally accepted dosing strategy is the use of a variable dose calculated using patient-specific characteristics as per manufacturer's instruction. However, evidence exists that the use of a fixed low dose of 1000 international units of factor IX (IU fIX) might also suffice. Recent studies indicate that in terms of haemostatic effectiveness, the fixed dosing strategy might be even superior to the variable dosing strategy. The PROPER3 (PROthrombin complex concentrate: Prospective Evaluation and Rationalisation, number 3) study aims to confirm the non-inferiority, and explore superiority, in haemostatic effectiveness of the fixed PCC dosing strategy compared with the variable dosing strategy in VKA-related extracranial bleeding emergencies.
Methods and analysis: The study is designed as a randomised controlled multicentre non-inferiority trial. Eligibility criteria are an indication for PCC due to VKA-related extracranial bleeding in subjects 18 years of age or older. The control group will receive a variable dose, determined by patient-specific bodyweight and international normalised ratio. The intervention group is dosed a fixed 1000 IU fIX PCC. Primary outcome is the haemostatic effectiveness of both treatments, as defined by the 2016 International Society on Thrombosis and Haemostasis (ISTH) criteria. The sample size is set at 155 patients per treatment arm, requiring 310 patients in total. Non-inferiority on the proportion (risk) difference of the primary outcome will be evaluated using the asymptotic Wald test for non-inferiority. The non-inferiority margin is set at 6%. The primary analysis will be based on the per-protocol population.
Ethics and dissemination: Study results will be published in an international journal, communicated to discipline-specific associations and presented at (inter)national meetings and congresses.
Trial registration number: EUCTR2014-000392-33; Pre-results.
Keywords: anticoagulation.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: KM reports grants from Sanquin during the conduct of the study and travel support, speaker fees or consulting fees from Baxter, Bayer, Sanquin, Pfizer, Boehringer Ingelheim, BMS, Aspen and Uniqure outside the submitted work.
Figures

References
-
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. - PubMed
-
- van der Meer FJ, Rosendaal FR, Vandenbroucke JP, et al. . Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Arch Intern Med 1993;153:1557–62. - PubMed
-
- Palareti G, Leali N, Coccheri S, et al. . Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996;348:423–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
