Performance of a Highly Sensitive Mycobacterium tuberculosis Complex Real-Time PCR Assay for Diagnosis of Pulmonary Tuberculosis in a Low-Prevalence Setting: a Prospective Intervention Study
- PMID: 29540457
- PMCID: PMC5925698
- DOI: 10.1128/JCM.00116-18
Performance of a Highly Sensitive Mycobacterium tuberculosis Complex Real-Time PCR Assay for Diagnosis of Pulmonary Tuberculosis in a Low-Prevalence Setting: a Prospective Intervention Study
Abstract
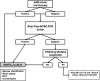
The potential impact of routine real-time PCR testing of respiratory specimens from patients with presumptive tuberculosis in terms of diagnostic accuracy and time to tuberculosis treatment inception in low-prevalence settings remains largely unexplored. We conducted a prospective intervention cohort study. Respiratory specimens from 1,020 patients were examined by acid-fast bacillus smear microscopy, tested by a real-time Mycobacterium tuberculosis complex PCR assay (Abbott RealTime MTB PCR), and cultured in mycobacterial media. Seventeen patients tested positive by PCR (5 were acid-fast bacillus smear positive and 12 acid-fast bacillus smear negative), and Mycobacterium tuberculosis was recovered from cultures for 12 of them. Patients testing positive by PCR and negative by culture (n = 5) were treated and deemed to have responded to antituberculosis therapy. There were no PCR-negative/culture-positive cases, and none of the patients testing positive for nontuberculous mycobacteria (n = 20) yielded a positive PCR result. The data indicated that routine testing of respiratory specimens from patients with presumptive tuberculosis by the RealTime MTB PCR assay improves the tuberculosis diagnostic yield and may reduce the time to antituberculosis treatment initiation. On the basis of our data, we propose a novel mycobacterial laboratory algorithm for tuberculosis diagnosis.
Keywords: Mycobacterium tuberculosis; acid-fast bacillus smear microscopy; mycobacterial culture; nontuberculous mycobacteria; real-time PCR.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Clinical evaluation of the Abbott RealTime MTB Assay for direct detection of Mycobacterium tuberculosis-complex from respiratory and non-respiratory samples.Tuberculosis (Edinb). 2017 May;104:65-69. doi: 10.1016/j.tube.2017.03.002. Epub 2017 Mar 3. Tuberculosis (Edinb). 2017. PMID: 28454651
-
Performance of the new automated Abbott RealTime MTB assay for rapid detection of Mycobacterium tuberculosis complex in respiratory specimens.Eur J Clin Microbiol Infect Dis. 2015 Sep;34(9):1827-32. doi: 10.1007/s10096-015-2419-5. Epub 2015 Jun 13. Eur J Clin Microbiol Infect Dis. 2015. PMID: 26071001
-
Evaluation of the Abbott RealTime MTB and RealTime MTB INH/RIF Assays for Direct Detection of Mycobacterium tuberculosis Complex and Resistance Markers in Respiratory and Extrapulmonary Specimens.J Clin Microbiol. 2016 Dec;54(12):3022-3027. doi: 10.1128/JCM.01144-16. Epub 2016 Oct 12. J Clin Microbiol. 2016. PMID: 27733630 Free PMC article.
-
Clinical application of the Mycobacterium tuberculosis direct test: case report, literature review, and proposed clinical algorithm.Chest. 1998 Jul;114(1):317-23. doi: 10.1378/chest.114.1.317. Chest. 1998. PMID: 9674487 Review.
-
Diagnostic accuracy of in-house real-time PCR assay for Mycobacterium tuberculosis: a systematic review and meta-analysis.BMC Infect Dis. 2019 Aug 8;19(1):701. doi: 10.1186/s12879-019-4273-z. BMC Infect Dis. 2019. PMID: 31395014 Free PMC article.
Cited by
-
Current progress of functional nanobiosensors for potential tuberculosis diagnosis: The novel way for TB control?Front Bioeng Biotechnol. 2022 Dec 15;10:1036678. doi: 10.3389/fbioe.2022.1036678. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36588948 Free PMC article.
-
Abbott realtime MTB assay for detecting Mycobacterium tuberculosis complex in respiratory specimens: a cost-benefit analysis.Eur J Clin Microbiol Infect Dis. 2024 Sep;43(9):1699-1709. doi: 10.1007/s10096-024-04880-1. Epub 2024 Jul 3. Eur J Clin Microbiol Infect Dis. 2024. PMID: 38958810
-
Evaluation of the STANDARD M10 MDR-TB and MTB/NTM assays for the detection of Mycobacterium tuberculosis, rifampicin and isoniazid resistance, and nontuberculous mycobacteria in a low-incidence setting.J Clin Microbiol. 2024 Oct 16;62(10):e0040224. doi: 10.1128/jcm.00402-24. Epub 2024 Sep 19. J Clin Microbiol. 2024. PMID: 39297626 Free PMC article.
-
Evaluation of a real-time PCR assay performance to detect Mycobacterium tuberculosis, rifampicin, and isoniazid resistance in sputum specimens: a multicenter study in two major cities of Indonesia.Front Microbiol. 2024 May 10;15:1372647. doi: 10.3389/fmicb.2024.1372647. eCollection 2024. Front Microbiol. 2024. PMID: 38800757 Free PMC article.
-
Two target genes based multiple cross displacement amplification combined with a lateral flow biosensor for the detection of Mycobacterium tuberculosis complex.BMC Microbiol. 2021 Oct 4;21(1):267. doi: 10.1186/s12866-021-02328-6. BMC Microbiol. 2021. PMID: 34607556 Free PMC article.
References
-
- Greenaway C, Menzies D, Fanning A, Grewal R, Yuan L, FitzGerald JM; Canadian Collaborative Group in nosocomial Transmission of Tuberculosis. 2002. Delay in diagnosis among hospitalized patients with active tuberculosis—predictors and outcomes. Am J Respir Crit Care Med 165:927–933. doi:10.1164/ajrccm.165.7.2107040. - DOI - PubMed
-
- Golub JE, Bur S, Cronin WA, Gange S, Baruch N, Comstock GW, Chaisson RE. 2006. Delayed tuberculosis diagnosis and tuberculosis transmission. Int J Tuberc Lung Dis 10:24–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

