An activating mutation of interferon regulatory factor 4 (IRF4) in adult T-cell leukemia
- PMID: 29540473
- PMCID: PMC5936815
- DOI: 10.1074/jbc.RA117.000164
An activating mutation of interferon regulatory factor 4 (IRF4) in adult T-cell leukemia
Abstract
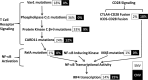
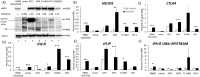
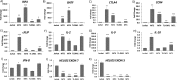
The human T-cell leukemia virus-1 (HTLV-1) oncoprotein Tax drives cell proliferation and resistance to apoptosis early in the pathogenesis of adult T-cell leukemia (ATL). Subsequently, probably as a result of specific immunoediting, Tax expression is down-regulated and functionally replaced by somatic driver mutations of the host genome. Both amplification and point mutations of interferon regulatory factor 4 (IRF4) have been previously detected in ATL., K59R is the most common single-nucleotide variation of IRF4 and is found exclusively in ATL. High-throughput whole-exome sequencing revealed recurrent activating genetic alterations in the T-cell receptor, CD28, and NF-κB pathways. We found that IRF4, which is transcriptionally activated downstream of these pathways, is frequently mutated in ATL. IRF4 RNA, protein, and IRF4 transcriptional targets are uniformly elevated in HTLV-1-transformed cells and ATL cell lines, and IRF4 was bound to genomic regulatory DNA of many of these transcriptional targets in HTLV-1-transformed cell lines. We further noted that the K59R IRF4 mutant is expressed at higher levels in the nucleus than WT IRF4 and is transcriptionally more active. Expression of both WT and the K59R mutant of IRF4 from a constitutive promoter in retrovirally transduced murine bone marrow cells increased the abundance of T lymphocytes but not myeloid cells or B lymphocytes in mice. IRF4 may represent a therapeutic target in ATL because ATL cells select for a mutant of IRF4 with higher nuclear expression and transcriptional activity, and overexpression of IRF4 induces the expansion of T lymphocytes in vivo.
Keywords: ATL; HTLV; NF-kappaB transcription factor; cancer biology; chromatin immunoprecipitation (ChiP); driver mutation; interferon regulatory factor (IRF); retrovirus.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation.Blood. 2000 Oct 15;96(8):2849-55. Blood. 2000. PMID: 11023521
-
CD69 overexpression by human T-cell leukemia virus type 1 Tax transactivation.Biochim Biophys Acta. 2013 Jun;1833(6):1542-52. doi: 10.1016/j.bbamcr.2013.03.006. Epub 2013 Mar 16. Biochim Biophys Acta. 2013. PMID: 23507197
-
Expression of human inducible nitric oxide synthase gene in T-cell lines infected with human T-cell leukemia virus type-I and primary adult T-cell leukemia cells.Blood. 1999 Oct 15;94(8):2862-70. Blood. 1999. PMID: 10515890
-
[Genetic analysis and its clinical implication in adult T-cell leukemia/lymphoma].Rinsho Ketsueki. 2018;59(10):2127-2135. doi: 10.11406/rinketsu.59.2127. Rinsho Ketsueki. 2018. PMID: 30305518 Review. Japanese.
-
Adult T-cell leukemia-lymphoma as a viral disease: Subtypes based on viral aspects.Cancer Sci. 2021 May;112(5):1688-1694. doi: 10.1111/cas.14869. Epub 2021 Mar 30. Cancer Sci. 2021. PMID: 33630351 Free PMC article. Review.
Cited by
-
Alvocidib inhibits IRF4 expression via super-enhancer suppression and adult T-cell leukemia/lymphoma cell growth.Cancer Sci. 2022 Dec;113(12):4092-4103. doi: 10.1111/cas.15550. Epub 2022 Oct 3. Cancer Sci. 2022. PMID: 36047964 Free PMC article.
-
The molecular basis for the development of adult T-cell leukemia/lymphoma in patients with an IRF4K59R mutation.Protein Sci. 2022 Apr;31(4):787-796. doi: 10.1002/pro.4260. Epub 2022 Feb 15. Protein Sci. 2022. PMID: 34913532 Free PMC article.
-
Interferon regulatory factor 4 as a therapeutic target in adult T-cell leukemia lymphoma.Retrovirology. 2020 Aug 28;17(1):27. doi: 10.1186/s12977-020-00535-z. Retrovirology. 2020. PMID: 32859220 Free PMC article.
-
Molecular biology of human T cell leukemia virus.Semin Diagn Pathol. 2020 Mar;37(2):104-109. doi: 10.1053/j.semdp.2019.04.003. Epub 2019 Apr 16. Semin Diagn Pathol. 2020. PMID: 31103249 Free PMC article. Review.
-
Novel mutations of SRPX facilitate the stemness and malignant progression of glioma.Br J Cancer. 2025 Jul 15. doi: 10.1038/s41416-025-03091-5. Online ahead of print. Br J Cancer. 2025. PMID: 40665012
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

