Transcription factor 21 (TCF21) promotes proinflammatory interleukin 6 expression and extracellular matrix remodeling in visceral adipose stem cells
- PMID: 29540474
- PMCID: PMC5925812
- DOI: 10.1074/jbc.RA117.000456
Transcription factor 21 (TCF21) promotes proinflammatory interleukin 6 expression and extracellular matrix remodeling in visceral adipose stem cells
Abstract
The visceral (VIS) and subcutaneous (SQ) fat pads are developmentally distinct white adipose tissue depots and contribute differently to inflammation and insulin resistance associated with obesity. The basic helix-loop-helix transcriptional regulator, transcription factor 21 (TCF21), is a marker gene for white adipose tissues and is abundantly expressed in VIS-derived adipose stem cells (ASCs), but not in SQ-derived ASCs. However, TCF21's role in regulating fat depot-specific gene expression and function is incompletely understood. Here, using siRNA-mediated Tcf21 knockdowns and lentiviral gene transfer of TCF21 in mouse ASCs, we demonstrate that TCF21 is required for the VIS ASC-specific expression of interleukin 6 (IL6), a key cytokine that contributes to the proinflammatory nature of VIS depots. Concurrently, TCF21 promotes MMP-dependent collagen degradation and type IV collagen deposition through the regulation of the extracellular matrix (ECM) modifiers, matrix metalloproteinase (MMP) 2, MMP13, and tissue inhibitor of MMP1 (TIMP1), as well as collagen type IV α1 chain (COL4A1) in VIS ASCs. We also found that although IL6 mediates the expression of Mmp13 and Timp1 in VIS ASCs, the TCF21-dependent expression of Mmp2 and Col4a1 is IL6-independent. These results suggest that TCF21 contributes to the proinflammatory environment in VIS fat depots and to active ECM remodeling of these depots by regulating IL6 expression and MMP-dependent ECM remodeling in a spatiotemporally coordinated manner.
Keywords: adipose tissue; collagen; interleukin 6 (IL6); matrix metalloproteinase (MMP); transcription.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
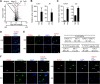


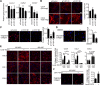
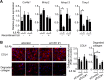
References
-
- Gesta S., Blüher M., Yamamoto Y., Norris A. W., Berndt J., Kralisch S., Boucher J., Lewis C., and Kahn C. R. (2006) Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. U.S.A. 103, 6676–6681 10.1073/pnas.0601752103 - DOI - PMC - PubMed
-
- Harman-Boehm I., Blüher M., Redel H., Sion-Vardy N., Ovadia S., Avinoach E., Shai I., Klöting N., Stumvoll M., Bashan N., and Rudich A. (2007) Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J. Clin. Endocrinol. Metab. 92, 2240–2247 10.1210/jc.2006-1811 - DOI - PubMed
-
- Bastard J. P., Maachi M., Van Nhieu J. T., Jardel C., Bruckert E., Grimaldi A., Robert J. J., Capeau J., and Hainque B. (2002) Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. Metab. 87, 2084–2089 10.1210/jcem.87.5.8450 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

