The cellular stress proteins CHCHD10 and MNRR1 (CHCHD2): Partners in mitochondrial and nuclear function and dysfunction
- PMID: 29540477
- PMCID: PMC5925800
- DOI: 10.1074/jbc.RA117.001073
The cellular stress proteins CHCHD10 and MNRR1 (CHCHD2): Partners in mitochondrial and nuclear function and dysfunction
Abstract
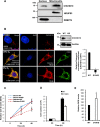
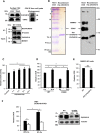
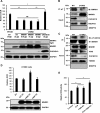
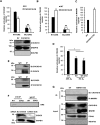
Coiled-coil-helix-coiled-coil-helix domain-containing 10 (CHCHD10) and CHCHD2 (MNRR1) are homologous proteins with 58% sequence identity and belong to the twin CX9C family of proteins that mediate cellular stress responses. Despite the identification of several neurodegeneration-associated mutations in the CHCHD10 gene, few studies have assessed its physiological role. Here, we investigated CHCHD10's function as a regulator of oxidative phosphorylation in the mitochondria and the nucleus. We show that CHCHD10 copurifies with cytochrome c oxidase (COX) and up-regulates COX activity by serving as a scaffolding protein required for MNRR1 phosphorylation, mediated by ARG (ABL proto-oncogene 2, nonreceptor tyrosine kinase (ABL2)). The CHCHD10 gene was maximally transcribed in cultured cells at 8% oxygen, unlike MNRR1, which was maximally expressed at 4%, suggesting a fine-tuned oxygen-sensing system that adapts to the varying oxygen concentrations in the human body under physiological conditions. We show that nuclear CHCHD10 protein down-regulates the expression of genes harboring the oxygen-responsive element (ORE) in their promoters by interacting with and augmenting the activity of the largely uncharacterized transcriptional repressor CXXC finger protein 5 (CXXC5). We further show that two genetic CHCHD10 disease variants, G66V and P80L, in the mitochondria exhibit faulty interactions with MNRR1 and COX, reducing respiration and increasing reactive oxygen species (ROS), and in the nucleus abrogating transcriptional repression of ORE-containing genes. Our results reveal that CHCHD10 positively regulates mitochondrial respiration and contributes to transcriptional repression of ORE-containing genes in the nucleus, and that genetic CHCHD10 variants are impaired in these activities.
Keywords: cell stress; cytochrome c oxidase (Complex IV); energy metabolism; hypoxia; mitochondria; mitochondrial disease; neurodegenerative disease; scaffolding protein; transcriptional regulator.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
MNRR1, a Biorganellar Regulator of Mitochondria.Oxid Med Cell Longev. 2017;2017:6739236. doi: 10.1155/2017/6739236. Epub 2017 Jun 8. Oxid Med Cell Longev. 2017. PMID: 28685009 Free PMC article. Review.
-
Abl2 kinase phosphorylates Bi-organellar regulator MNRR1 in mitochondria, stimulating respiration.Biochim Biophys Acta Mol Cell Res. 2017 Feb;1864(2):440-448. doi: 10.1016/j.bbamcr.2016.11.029. Epub 2016 Nov 30. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27913209
-
MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism.Mitochondrion. 2015 Jan;20:43-51. doi: 10.1016/j.mito.2014.10.003. Epub 2014 Oct 12. Mitochondrion. 2015. PMID: 25315652
-
In vitro and in vivo studies of the ALS-FTLD protein CHCHD10 reveal novel mitochondrial topology and protein interactions.Hum Mol Genet. 2018 Jan 1;27(1):160-177. doi: 10.1093/hmg/ddx397. Hum Mol Genet. 2018. PMID: 29112723 Free PMC article.
-
Twin CHCH Proteins, CHCHD2, and CHCHD10: Key Molecules of Parkinson's Disease, Amyotrophic Lateral Sclerosis, and Frontotemporal Dementia.Int J Mol Sci. 2019 Feb 20;20(4):908. doi: 10.3390/ijms20040908. Int J Mol Sci. 2019. PMID: 30791515 Free PMC article. Review.
Cited by
-
Examining the relationship between astrocyte dysfunction and neurodegeneration in ALS using hiPSCs.Neurobiol Dis. 2019 Dec;132:104562. doi: 10.1016/j.nbd.2019.104562. Epub 2019 Aug 2. Neurobiol Dis. 2019. PMID: 31381978 Free PMC article. Review.
-
CHCHD2 Regulates Mitochondrial Function and Apoptosis of Ectopic Endometrial Stromal Cells in the Pathogenesis of Endometriosis.Reprod Sci. 2022 Aug;29(8):2152-2164. doi: 10.1007/s43032-021-00831-9. Epub 2022 Feb 14. Reprod Sci. 2022. PMID: 35157262
-
Botanical Drug Puerarin Promotes Neuronal Survival and Neurite Outgrowth against MPTP/MPP+-Induced Toxicity via Progesterone Receptor Signaling.Oxid Med Cell Longev. 2020 Oct 17;2020:7635291. doi: 10.1155/2020/7635291. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33123315 Free PMC article.
-
Mitochondrial CHCHD2: Disease-Associated Mutations, Physiological Functions, and Current Animal Models.Front Aging Neurosci. 2021 Apr 22;13:660843. doi: 10.3389/fnagi.2021.660843. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33967741 Free PMC article. Review.
-
Lipopolysaccharide induces placental mitochondrial dysfunction in murine and human systems by reducing MNRR1 levels via a TLR4-independent pathway.iScience. 2022 Oct 12;25(11):105342. doi: 10.1016/j.isci.2022.105342. eCollection 2022 Nov 18. iScience. 2022. PMID: 36339251 Free PMC article.
References
-
- Chacinska A., Pfannschmidt S., Wiedemann N., Kozjak V., Sanjuán Szklarz L. K., Schulze-Specking A., Truscott K. N., Guiard B., Meisinger C., and Pfanner N. (2004) Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 23, 3735–3746 10.1038/sj.emboj.7600389 - DOI - PMC - PubMed
-
- Darshi M., Mendiola V. L., Mackey M. R., Murphy A. N., Koller A., Perkins G. A., Ellisman M. H., and Taylor S. S. (2011) ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J. Biol. Chem. 286, 2918–2932 10.1074/jbc.M110.171975 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

