Pharmacologically targeting the myristoylation of the scaffold protein FRS2α inhibits FGF/FGFR-mediated oncogenic signaling and tumor progression
- PMID: 29540482
- PMCID: PMC5925806
- DOI: 10.1074/jbc.RA117.000940
Pharmacologically targeting the myristoylation of the scaffold protein FRS2α inhibits FGF/FGFR-mediated oncogenic signaling and tumor progression
Abstract
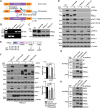
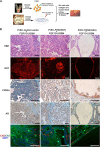
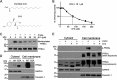
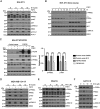
Fibroblast growth factor (FGF)/FGF receptor (FGFR) signaling facilitates tumor initiation and progression. Although currently approved inhibitors of FGFR kinase have shown therapeutic benefit in clinical trials, overexpression or mutations of FGFRs eventually confer drug resistance and thereby abrogate the desired activity of kinase inhibitors in many cancer types. In this study, we report that loss of myristoylation of fibroblast growth factor receptor substrate 2 (FRS2α), a scaffold protein essential for FGFR signaling, inhibits FGF/FGFR-mediated oncogenic signaling and FGF10-induced tumorigenesis. Moreover, a previously synthesized myristoyl-CoA analog, B13, which targets the activity of N-myristoyltransferases, suppressed FRS2α myristoylation and decreased the phosphorylation with mild alteration of FRS2α localization at the cell membrane. B13 inhibited oncogenic signaling induced by WT FGFRs or their drug-resistant mutants (FGFRsDRM). B13 alone or in combination with an FGFR inhibitor suppressed FGF-induced WT FGFR- or FGFRDRM-initiated phosphoinositide 3-kinase (PI3K) activity or MAPK signaling, inducing cell cycle arrest and thereby inhibiting cell proliferation and migration in several cancer cell types. Finally, B13 significantly inhibited the growth of xenograft tumors without pathological toxicity to the liver, kidney, or lung in vivo In summary, our study suggests a possible therapeutic approach for inhibiting FGF/FGFR-mediated cancer progression and drug-resistant FGF/FGFR mutants.
Keywords: B13; FRS2; cancer; drug action; fibroblast growth factor (FGF); fibroblast growth factor receptor (FGFR); myristoylation; protein acylation.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Dieci M. V., Arnedos M., Andre F., and Soria J. C. (2013) Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 3, 264–279 10.1158/2159-8290.CD-12-0362 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

