Ampicillin permeation across OmpF, the major outer-membrane channel in Escherichia coli
- PMID: 29540483
- PMCID: PMC5936826
- DOI: 10.1074/jbc.RA117.000705
Ampicillin permeation across OmpF, the major outer-membrane channel in Escherichia coli
Abstract
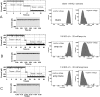
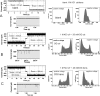
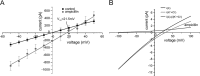
The outer cell wall of the Gram-negative bacteria is a crucial barrier for antibiotics to reach their target. Here, we show that the chemical stability of the widely used antibiotic ampicillin is a major factor in the permeation across OmpF to reach the target in the periplasm. Using planar lipid bilayers we investigated the interactions and permeation of OmpF with ampicillin, its basic pH-induced primary degradation product (penicilloic acid), and the chemically more stable benzylpenicillin. We found that the solute-induced ion current fluctuation is 10 times higher with penicilloic acid than with ampicillin. Furthermore, we also found that ampicillin can easily permeate through OmpF, at an ampicillin gradient of 10 μm and a conductance of Gamp ≅ 3.8 fS, with a flux rate of roughly 237 molecules/s of ampicillin at Vm = 10 mV. The structurally related benzylpenicillin yields a lower conductance of Gamp ≅ 2 fS, corresponding to a flux rate of ≈120 molecules/s. In contrast, the similar sized penicilloic acid was nearly unable to permeate through OmpF. MD calculations show that, besides their charge difference, the main differences between ampicillin and penicilloic acid are the shape of the molecules, and the strength and direction of the dipole vector. Our results show that OmpF can impose selective permeation on similar sized molecules based on their structure and their dipolar properties.
Keywords: NMR; OmpF; ampicillin; antibiotics; bacteria; electrophysiology; membrane reconstitution; membrane transport; peniciollic acid; planar bilayer.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Similar articles
-
Permeation of antibiotics through Escherichia coli OmpF and OmpC porins: screening for influx on a single-molecule level.J Biomol Screen. 2010 Mar;15(3):302-7. doi: 10.1177/1087057109357791. Epub 2010 Jan 19. J Biomol Screen. 2010. PMID: 20086208
-
Breaching the Barrier: Genome-Wide Investigation into the Role of a Primary Amine in Promoting E. coli Outer-Membrane Passage and Growth Inhibition by Ampicillin.Microbiol Spectr. 2022 Dec 21;10(6):e0359322. doi: 10.1128/spectrum.03593-22. Epub 2022 Nov 21. Microbiol Spectr. 2022. PMID: 36409154 Free PMC article.
-
Toward screening for antibiotics with enhanced permeation properties through bacterial porins.Biochemistry. 2010 Aug 17;49(32):6928-35. doi: 10.1021/bi100845x. Biochemistry. 2010. PMID: 20604536
-
Rapid screening of membrane protein activity: electrophysiological analysis of OmpF reconstituted in proteoliposomes.Lab Chip. 2008 Apr;8(4):587-95. doi: 10.1039/b713982a. Epub 2008 Feb 15. Lab Chip. 2008. PMID: 18369514
-
Molecular basis of voltage gating of OmpF porin.Biochem Cell Biol. 2002;80(5):517-23. doi: 10.1139/o02-145. Biochem Cell Biol. 2002. PMID: 12440693 Review.
Cited by
-
An In Vitro System Mimics the Intestinal Microbiota of Striped Beakfish (Oplegnathus fasciatus) and Inhibits Vibrio alginolyticus by Limosilactobacillus reuteri-Derived Extracellular Vesicles.Animals (Basel). 2024 Jun 14;14(12):1792. doi: 10.3390/ani14121792. Animals (Basel). 2024. PMID: 38929411 Free PMC article.
-
β-lactam resistance associated with β-lactamase production and porin alteration in clinical isolates of E. coli and K. pneumoniae.PLoS One. 2021 May 20;16(5):e0251594. doi: 10.1371/journal.pone.0251594. eCollection 2021. PLoS One. 2021. PMID: 34014957 Free PMC article.
-
The Whole Is Bigger than the Sum of Its Parts: Drug Transport in the Context of Two Membranes with Active Efflux.Chem Rev. 2021 May 12;121(9):5597-5631. doi: 10.1021/acs.chemrev.0c01137. Epub 2021 Feb 17. Chem Rev. 2021. PMID: 33596653 Free PMC article.
-
Cephalosporin translocation across enterobacterial OmpF and OmpC channels, a filter across the outer membrane.Commun Biol. 2022 Oct 5;5(1):1059. doi: 10.1038/s42003-022-04035-y. Commun Biol. 2022. PMID: 36198902 Free PMC article.
-
Establishment and evaluation of a specific antibiotic-induced inflammatory bowel disease model in rats.PLoS One. 2022 Feb 22;17(2):e0264194. doi: 10.1371/journal.pone.0264194. eCollection 2022. PLoS One. 2022. PMID: 35192646 Free PMC article.
References
-
- Collignon P. C., Conly J. M., Andremont A., McEwen S. A., Aidara-Kane A., World Health Organization Advisory Group, Bogata Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO-AGISR), Agerso Y., Andremont A., Collignon P., Conly J., Dang Ninh T., Donado-Godoy P., Fedorda-Cray P., Fernandez H., et al. (2016) World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin. Infect. Dis. 63, 1087–1093 10.1093/cid/ciw475 - DOI - PubMed
-
- Gallelli J. F. (1967) Stability studies of drugs used in intravenous solutions. I. Am. J. Hosp. Pharm. 24, 425–433 - PubMed
-
- Nguyen-Disteche M., Pollock J. J., Ghuysen J. M., Puig J., Reynolds P., Perkins H. R., Coyette J., and Salton M. R. (1974) Sensitivity to ampicillin and cephalothin of enzymes involved in wall peptide crosslinking in Escherichia coli K12, strain 44. Eur. J. Biochem. 41, 457–463 10.1111/j.1432-1033.1974.tb03287.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

