Adaptive remodeling of skeletal muscle energy metabolism in high-altitude hypoxia: Lessons from AltitudeOmics
- PMID: 29540485
- PMCID: PMC5936810
- DOI: 10.1074/jbc.RA117.000470
Adaptive remodeling of skeletal muscle energy metabolism in high-altitude hypoxia: Lessons from AltitudeOmics
Abstract
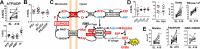
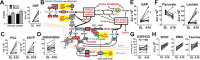
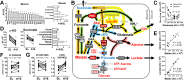
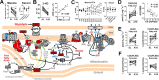
Metabolic responses to hypoxia play important roles in cell survival strategies and disease pathogenesis in humans. However, the homeostatic adjustments that balance changes in energy supply and demand to maintain organismal function under chronic low oxygen conditions remain incompletely understood, making it difficult to distinguish adaptive from maladaptive responses in hypoxia-related pathologies. We integrated metabolomic and proteomic profiling with mitochondrial respirometry and blood gas analyses to comprehensively define the physiological responses of skeletal muscle energy metabolism to 16 days of high-altitude hypoxia (5260 m) in healthy volunteers from the AltitudeOmics project. In contrast to the view that hypoxia down-regulates aerobic metabolism, results show that mitochondria play a central role in muscle hypoxia adaptation by supporting higher resting phosphorylation potential and enhancing the efficiency of long-chain acylcarnitine oxidation. This directs increases in muscle glucose toward pentose phosphate and one-carbon metabolism pathways that support cytosolic redox balance and help mitigate the effects of increased protein and purine nucleotide catabolism in hypoxia. Muscle accumulation of free amino acids favor these adjustments by coordinating cytosolic and mitochondrial pathways to rid the cell of excess nitrogen, but might ultimately limit muscle oxidative capacity in vivo Collectively, these studies illustrate how an integration of aerobic and anaerobic metabolism is required for physiological hypoxia adaptation in skeletal muscle, and highlight protein catabolism and allosteric regulation as unexpected orchestrators of metabolic remodeling in this context. These findings have important implications for the management of hypoxia-related diseases and other conditions associated with chronic catabolic stress.
Keywords: anaplerosis; beta-oxidation; bioenergetics; fatty acid oxidation; glycolysis; hypoxia; mitochondrial metabolism; one-carbon metabolism; oxidation-reduction (redox).
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
E. G. is Chief Executive Officer of Oroboros Instruments, Innsbruck, Austria
Figures


References
-
- Meyer A., Zoll J., Charles A. L., Charloux A., de Blay F., Diemunsch P., Sibilia J., Piquard F., and Geny B. (2013) Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: central actor and therapeutic target. Exp. Physiol. 98, 1063–1078 10.1113/expphysiol.2012.069468 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

