Control of (pre)-analytical aspects in immunoassay measurements of metabolic hormones in rodents
- PMID: 29540488
- PMCID: PMC5881432
- DOI: 10.1530/EC-18-0035
Control of (pre)-analytical aspects in immunoassay measurements of metabolic hormones in rodents
Abstract
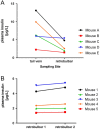
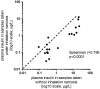
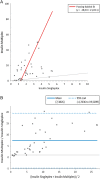
The measurement of circulating hormones by immunoassay remains a cornerstone in preclinical endocrine research. For scientists conducting and interpreting immunoassay measurements of rodent samples, the paramount aim usually is to obtain reliable and meaningful measurement data in order to draw conclusions on biological processes. However, the biological variability between samples is not the only variable affecting the readout of an immunoassay measurement and a considerable amount of unwanted or unintended variability can be quickly introduced during the pre-analytical and analytical phase. This review aims to increase the awareness for the factors 'pre-analytical' and 'analytical' variability particularly in the context of immunoassay measurement of circulating metabolic hormones in rodent samples. In addition, guidance is provided how to gain control over these variables and how to avoid common pitfalls associated with sample collection, processing, storage and measurement. Furthermore, recommendations are given on how to perform a basic validation of novel single and multiplex immunoassays for the measurement of metabolic hormones in rodents. Finally, practical examples from immunoassay measurements of plasma insulin in mice address the factors 'sampling site and inhalation anesthesia' as frequent sources of introducing an unwanted variability during the pre-analytical phase. The knowledge about the influence of both types of variability on the immunoassay measurement of circulating hormones as well as strategies to control these variables are crucial, on the one hand, for planning and realization of metabolic rodent studies and, on the other hand, for the generation and interpretation of meaningful immunoassay data from rodent samples.
Keywords: ELISA; assay performance; assay validation; endocrine factors; immunoassay; insulin; metabolism; mice; multiplex; plasma hormones; variability.
© 2018 The authors.
Figures
References
-
- Morgan CR, Lazarow A. Immunoassay of insulin: two antibody system: plasma insulin levels of normal, subdiabetic and diabetic rats. Diabetes 1963. 12 115–126. ( 10.2337/diab.12.2.115) - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

