Transmission of microRNA antimiRs to mouse offspring via the maternal-placental-fetal unit
- PMID: 29540511
- PMCID: PMC5959254
- DOI: 10.1261/rna.063206.117
Transmission of microRNA antimiRs to mouse offspring via the maternal-placental-fetal unit
Abstract
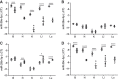
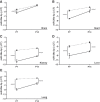
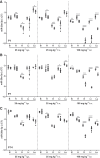
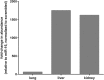
The emergence of microRNA as regulators of organogenesis and tissue differentiation has stimulated interest in the ablation of microRNA expression and function during discrete periods of development. To this end, inducible, conditional modulation of microRNA expression with doxycycline-based tetracycline-controlled transactivator and tamoxifen-based estrogen receptor systems has found widespread use. However, the induction agents and components of genome recombination systems negatively impact pregnancy, parturition, and postnatal development; thereby limiting the use of these technologies between late gestation and the early postnatal period. MicroRNA inhibitor (antimiR) administration also represents a means of neutralizing microRNA function in vitro and in vivo. To date, these studies have used direct (parenteral) administration of antimiRs to experimental animals. As an extension of this approach, an alternative means of regulating microRNA expression and function is described here: the maternal-placental-fetal transmission of antimiRs. When administered to pregnant dams, antimiRs were detected in offspring and resulted in a pronounced and persistent reduction in detectable steady-state free microRNA levels in the heart, kidney, liver, lungs, and brain. This effect was comparable to direct injection of newborn mouse pups with antimiRs, although maternal delivery resulted in fewer off-target effects. Furthermore, depletion of steady-state microRNA levels via the maternal route resulted in concomitant increases in steady-state levels of selected microRNA targets. This novel methodology permits the temporal regulation of microRNA function during late gestation and in neonates, without recourse to conventional approaches that rely on doxycycline and tamoxifen, which may confound studies on developmental processes.
Keywords: antimiR; development; maternal transmission; microRNA; organogenesis.
© 2018 Hönig et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures





Similar articles
-
Exposure of pregnant mice to carbon black by intratracheal instillation: toxicogenomic effects in dams and offspring.Mutat Res. 2012 Jun 14;745(1-2):73-83. doi: 10.1016/j.mrgentox.2011.09.018. Epub 2011 Oct 6. Mutat Res. 2012. PMID: 22001195
-
Estimation of placental and lactational transfer and tissue distribution of atrazine and its main metabolites in rodent dams, fetuses, and neonates with physiologically based pharmacokinetic modeling.Toxicol Appl Pharmacol. 2013 Nov 15;273(1):140-58. doi: 10.1016/j.taap.2013.08.010. Epub 2013 Aug 17. Toxicol Appl Pharmacol. 2013. PMID: 23958493
-
Actions of placental and fetal adrenal steroid hormones in primate pregnancy.Endocr Rev. 1995 Oct;16(5):608-48. doi: 10.1210/edrv-16-5-608. Endocr Rev. 1995. PMID: 8529574 Review.
-
Maternal and fetal genomes interplay through phosphoinositol 3-kinase(PI3K)-p110α signaling to modify placental resource allocation.Proc Natl Acad Sci U S A. 2016 Oct 4;113(40):11255-11260. doi: 10.1073/pnas.1602012113. Epub 2016 Sep 12. Proc Natl Acad Sci U S A. 2016. PMID: 27621448 Free PMC article.
-
Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development--a review.Placenta. 2002 Apr;23 Suppl A:S9-19. doi: 10.1053/plac.2002.0771. Placenta. 2002. PMID: 11978055 Review.
Cited by
-
The function of miRNAs in the process of kidney development.Noncoding RNA Res. 2023 Aug 23;8(4):593-601. doi: 10.1016/j.ncrna.2023.08.009. eCollection 2023 Dec. Noncoding RNA Res. 2023. PMID: 37680850 Free PMC article. Review.
-
Targeting miR-34a/Pdgfra interactions partially corrects alveologenesis in experimental bronchopulmonary dysplasia.EMBO Mol Med. 2019 Mar;11(3):e9448. doi: 10.15252/emmm.201809448. EMBO Mol Med. 2019. PMID: 30770339 Free PMC article.
References
-
- Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, et al. 2007. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549. - PubMed
-
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
