Predicting the effects of parasite co-infection across species boundaries
- PMID: 29540516
- PMCID: PMC5879626
- DOI: 10.1098/rspb.2017.2610
Predicting the effects of parasite co-infection across species boundaries
Abstract
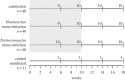
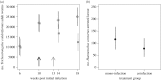
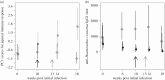
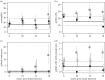
It is normal for hosts to be co-infected by parasites. Interactions among co-infecting species can have profound consequences, including changing parasite transmission dynamics, altering disease severity and confounding attempts at parasite control. Despite the importance of co-infection, there is currently no way to predict how different parasite species may interact with one another, nor the consequences of those interactions. Here, we demonstrate a method that enables such prediction by identifying two nematode parasite groups based on taxonomy and characteristics of the parasitological niche. From an understanding of the interactions between the two defined groups in one host system (wild rabbits), we predict how two different nematode species, from the same defined groups, will interact in co-infections in a different host system (sheep), and then we test this experimentally. We show that, as predicted, in co-infections, the blood-feeding nematode Haemonchus contortus suppresses aspects of the sheep immune response, thereby facilitating the establishment and/or survival of the nematode Trichostrongylus colubriformis; and that the T. colubriformis-induced immune response negatively affects H. contortus This work is, to our knowledge, the first to use empirical data from one host system to successfully predict the specific outcome of a different co-infection in a second host species. The study therefore takes the first step in defining a practical framework for predicting interspecific parasite interactions in other animal systems.
Keywords: co-infection; helminths; immune response; immunomodulation; nematode; population dynamics.
© 2018 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Haemonchus contortus and Trichostrongylus colubriformis did not adapt to long-term exposure to sheep that were genetically resistant or susceptible to nematode infections.Int J Parasitol. 2009 Apr;39(5):607-14. doi: 10.1016/j.ijpara.2008.08.013. Epub 2008 Nov 6. Int J Parasitol. 2009. PMID: 19027020
-
High genetic correlation between resistance to Haemonchus contortus and to Trichostrongylus colubriformis in INRA 401 sheep.Vet Parasitol. 2004 Jan 5;119(1):51-8. doi: 10.1016/j.vetpar.2003.10.014. Vet Parasitol. 2004. PMID: 15036576
-
Expression of genes in gastrointestinal and lymphatic tissues during parasite infection in sheep genetically resistant or susceptible to Trichostrongylus colubriformis and Haemonchus contortus.Int J Parasitol. 2010 Mar 15;40(4):417-29. doi: 10.1016/j.ijpara.2009.09.007. Epub 2009 Oct 13. Int J Parasitol. 2010. PMID: 19825375
-
The immunogenetics of resistance to Trichostrongylus colubriformis and Haemonchus contortus parasites in sheep.Br Vet J. 1995 Mar-Apr;151(2):119-40. doi: 10.1016/s0007-1935(95)80004-2. Br Vet J. 1995. PMID: 8920110 Review.
-
Ecology of the free-living stages of major trichostrongylid parasites of sheep.Vet Parasitol. 2006 Nov 30;142(1-2):1-15. doi: 10.1016/j.vetpar.2006.08.035. Epub 2006 Sep 29. Vet Parasitol. 2006. PMID: 17011129 Review.
Cited by
-
Infection against infection: parasite antagonism against parasites, viruses and bacteria.Infect Dis Poverty. 2019 Jun 15;8(1):49. doi: 10.1186/s40249-019-0560-6. Infect Dis Poverty. 2019. PMID: 31200765 Free PMC article. Review.
-
Detecting parasite associations within multi-species host and parasite communities.Proc Biol Sci. 2019 Oct 9;286(1912):20191109. doi: 10.1098/rspb.2019.1109. Epub 2019 Oct 2. Proc Biol Sci. 2019. PMID: 31575371 Free PMC article.
-
Characterising interactions between co-infecting parasites using age-intensity profiles.Int J Parasitol. 2020 Jan;50(1):23-26. doi: 10.1016/j.ijpara.2019.11.001. Epub 2019 Dec 14. Int J Parasitol. 2020. PMID: 31846621 Free PMC article.
-
Investigating the outcomes of virus coinfection within and across host species.PLoS Pathog. 2023 May 22;19(5):e1011044. doi: 10.1371/journal.ppat.1011044. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37216391 Free PMC article.
-
Toxocariasis in Ghanaian neighbourhoods: a need for action.Sci One Health. 2023 Jun 16;2:100018. doi: 10.1016/j.soh.2023.100018. eCollection 2023. Sci One Health. 2023. PMID: 39077038 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

