Regulation of Nociceptive Glutamatergic Signaling by Presynaptic Kv3.4 Channels in the Rat Spinal Dorsal Horn
- PMID: 29540546
- PMCID: PMC5895996
- DOI: 10.1523/JNEUROSCI.3212-17.2018
Regulation of Nociceptive Glutamatergic Signaling by Presynaptic Kv3.4 Channels in the Rat Spinal Dorsal Horn
Abstract
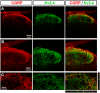
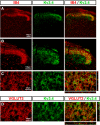
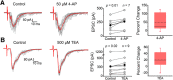
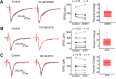
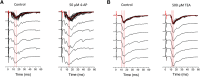
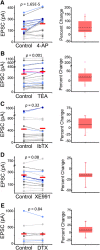
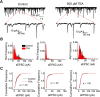
Presynaptic voltage-gated K+ (Kv) channels in dorsal root ganglion (DRG) neurons are thought to regulate nociceptive synaptic transmission in the spinal dorsal horn. However, the Kv channel subtypes responsible for this critical role have not been identified. The Kv3.4 channel is particularly important because it is robustly expressed in DRG nociceptors, where it regulates action potential (AP) duration. Furthermore, Kv3.4 dysfunction is implicated in the pathophysiology of neuropathic pain in multiple pain models. We hypothesized that, through their ability to modulate AP repolarization, Kv3.4 channels in DRG nociceptors help to regulate nociceptive synaptic transmission. To test this hypothesis, we investigated Kv3.4 immunoreactivity (IR) in the rat cervical superficial dorsal horn (sDH) in both sexes and implemented an intact spinal cord preparation to investigate glutamatergic synaptic currents from second order neurons in the sDH under conditions that selectively inhibit the Kv3.4 current. We found presynaptic Kv3.4 IR in peptidergic and nonpeptidergic nociceptive fibers of the sDH. The Kv3.4 channel is hypersensitive to 4-aminopyridine and tetraethylammonium (TEA). Accordingly, 50 μm 4-aminopyridine and 500 μm TEA significantly prolong the AP, slow the maximum rate of repolarization in small-diameter DRG neurons, and potentiate monosynaptic excitatory postsynaptic currents (EPSCs) in dorsal horn laminae I and II through a presynaptic mechanism. In contrast, highly specific inhibitors of BK, Kv7, and Kv1 channels are less effective modulators of the AP and have little to no effect on EPSCs. The results strongly suggest that presynaptic Kv3.4 channels are major regulators of nociceptive synaptic transmission in the spinal cord.SIGNIFICANCE STATEMENT Intractable neuropathic pain can result from disease or traumatic injury and many studies have been conducted to determine the underlying pathophysiological changes. Voltage-gated ion channels, including the K+ channel Kv3.4, are dysregulated in multiple pain models. Kv3.4 channels are ubiquitously expressed in the dorsal root ganglion (DRG), where they are major regulators of DRG excitability. However, little is known about the ionic mechanisms that regulate nociceptive synaptic transmission at the level of the first synapse in the spinal cord, which is critical to pain transmission in both intact and pathological states. Here, we show that Kv3.4 channels have a significant impact on glutamatergic synaptic transmission in the dorsal horn, further illuminating its potential as a molecular pain therapeutic target.
Keywords: Kv channel; pain transduction; spinal cord; synaptic transmission.
Copyright © 2018 the authors 0270-6474/18/383729-12$15.00/0.
Figures




Similar articles
-
Tunable Action Potential Repolarization Governed by Kv3.4 Channels in Dorsal Root Ganglion Neurons.J Neurosci. 2022 Nov 16;42(46):8647-8657. doi: 10.1523/JNEUROSCI.1210-22.2022. J Neurosci. 2022. PMID: 36198500 Free PMC article.
-
Calcineurin Dysregulation Underlies Spinal Cord Injury-Induced K+ Channel Dysfunction in DRG Neurons.J Neurosci. 2017 Aug 23;37(34):8256-8272. doi: 10.1523/JNEUROSCI.0434-17.2017. Epub 2017 Jul 27. J Neurosci. 2017. PMID: 28751455 Free PMC article.
-
Dysregulation of Kv3.4 channels in dorsal root ganglia following spinal cord injury.J Neurosci. 2015 Jan 21;35(3):1260-73. doi: 10.1523/JNEUROSCI.1594-14.2015. J Neurosci. 2015. PMID: 25609640 Free PMC article.
-
Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons.Int J Mol Sci. 2025 Mar 6;26(5):2356. doi: 10.3390/ijms26052356. Int J Mol Sci. 2025. PMID: 40076973 Free PMC article. Review.
-
Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain.Cell Mol Life Sci. 2019 May;76(10):1889-1899. doi: 10.1007/s00018-019-03047-y. Epub 2019 Feb 20. Cell Mol Life Sci. 2019. PMID: 30788514 Free PMC article. Review.
Cited by
-
Motor cortex analogue neurons in songbirds utilize Kv3 channels to generate ultranarrow spikes.Elife. 2023 May 9;12:e81992. doi: 10.7554/eLife.81992. Elife. 2023. PMID: 37158590 Free PMC article.
-
A high-affinity, partial antagonist effect of 3,4-diaminopyridine mediates action potential broadening and enhancement of transmitter release at NMJs.J Biol Chem. 2021 Jan-Jun;296:100302. doi: 10.1016/j.jbc.2021.100302. Epub 2021 Jan 17. J Biol Chem. 2021. PMID: 33465376 Free PMC article.
-
Structural insights into the function, dysfunction and modulation of Kv3 channels.Neuropharmacology. 2025 Sep 1;275:110483. doi: 10.1016/j.neuropharm.2025.110483. Epub 2025 Apr 25. Neuropharmacology. 2025. PMID: 40288604 Free PMC article. Review.
-
Distribution of Kv3 Subunits in Cochlear Afferent and Efferent Nerve Fibers Implies Distinct Role in Auditory Processing.Exp Neurobiol. 2020 Oct 31;29(5):344-355. doi: 10.5607/en20043. Exp Neurobiol. 2020. PMID: 33154197 Free PMC article.
-
K+ Channels in Primary Afferents and Their Role in Nerve Injury-Induced Pain.Front Cell Neurosci. 2020 Sep 17;14:566418. doi: 10.3389/fncel.2020.566418. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33093824 Free PMC article. Review.
References
-
- Beekwilder JP, O'Leary ME, van den Broek LP, van Kempen GT, Ypey DL, van den Berg RJ (2003) Kv1.1 channels of dorsal root ganglion neurons are inhibited by n-butyl-p-aminobenzoate, a promising anesthetic for the treatment of chronic pain. J Pharmacol Exp Ther 304:531–538. 10.1124/jpet.102.042135 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
