JIP1-Mediated JNK Activation Negatively Regulates Synaptic Plasticity and Spatial Memory
- PMID: 29540552
- PMCID: PMC5895995
- DOI: 10.1523/JNEUROSCI.1913-17.2018
JIP1-Mediated JNK Activation Negatively Regulates Synaptic Plasticity and Spatial Memory
Abstract
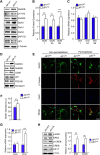
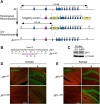
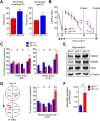
The c-Jun N-terminal kinase (JNK) signal transduction pathway is implicated in learning and memory. Here, we examined the role of JNK activation mediated by the JNK-interacting protein 1 (JIP1) scaffold protein. We compared male wild-type mice with a mouse model harboring a point mutation in the Jip1 gene that selectively blocks JIP1-mediated JNK activation. These male mutant mice exhibited increased NMDAR currents, increased NMDAR-mediated gene expression, and a lower threshold for induction of hippocampal long-term potentiation. The JIP1 mutant mice also displayed improved hippocampus-dependent spatial memory and enhanced associative fear conditioning. These results were confirmed using a second JIP1 mutant mouse model that suppresses JNK activity. Together, these observations establish that JIP1-mediated JNK activation contributes to the regulation of hippocampus-dependent, NMDAR-mediated synaptic plasticity and learning.SIGNIFICANCE STATEMENT The results of this study demonstrate that c-Jun N-terminal kinase (JNK) activation induced by the JNK-interacting protein 1 (JIP1) scaffold protein negatively regulates the threshold for induction of long-term synaptic plasticity through the NMDA-type glutamate receptor. This change in plasticity threshold influences learning. Indeed, mice with defects in JIP1-mediated JNK activation display enhanced memory in hippocampus-dependent tasks, such as contextual fear conditioning and Morris water maze, indicating that JIP1-JNK constrains spatial memory. This study identifies JIP1-mediated JNK activation as a novel molecular pathway that negatively regulates NMDAR-dependent synaptic plasticity and memory.
Keywords: JIP1; JNK; LTP; fear; memory; plasticity.
Copyright © 2018 the authors 0270-6474/18/383708-21$15.00/0.
Figures






Similar articles
-
Requirement of JIP1-mediated c-Jun N-terminal kinase activation for obesity-induced insulin resistance.Mol Cell Biol. 2010 Oct;30(19):4616-25. doi: 10.1128/MCB.00585-10. Epub 2010 Aug 2. Mol Cell Biol. 2010. PMID: 20679483 Free PMC article.
-
JIP1 regulates neuronal apoptosis in response to stress.Brain Res Mol Brain Res. 2005 Apr 4;134(2):282-93. doi: 10.1016/j.molbrainres.2004.10.039. Brain Res Mol Brain Res. 2005. PMID: 15836924
-
Akt1 regulates a JNK scaffold during excitotoxic apoptosis.Neuron. 2002 Aug 15;35(4):697-709. doi: 10.1016/s0896-6273(02)00821-8. Neuron. 2002. PMID: 12194869
-
Plasticity, hippocampal place cells, and cognitive maps.Arch Neurol. 2001 Jun;58(6):874-81. doi: 10.1001/archneur.58.6.874. Arch Neurol. 2001. PMID: 11405801 Review.
-
c-Jun N-terminal kinases in memory and synaptic plasticity.Rev Neurosci. 2011;22(4):403-10. doi: 10.1515/RNS.2011.032. Epub 2011 May 24. Rev Neurosci. 2011. PMID: 21605011 Review.
Cited by
-
Acupuncture Alleviates Chronic Ischemic White Matter Injury in SHR Rats via JNK-NMDAR Circuit.Mol Neurobiol. 2024 Jun;61(6):3144-3160. doi: 10.1007/s12035-023-03759-0. Epub 2023 Nov 17. Mol Neurobiol. 2024. PMID: 37976026
-
Involvement of JNK1 in Neuronal Polarization During Brain Development.Cells. 2020 Aug 13;9(8):1897. doi: 10.3390/cells9081897. Cells. 2020. PMID: 32823764 Free PMC article. Review.
-
JIP1 Deficiency Protects Retinal Ganglion Cells From Apoptosis in a Rotenone-Induced Injury Model.Front Cell Dev Biol. 2019 Oct 15;7:225. doi: 10.3389/fcell.2019.00225. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31681759 Free PMC article.
-
ALWPs Improve Cognitive Function and Regulate Aβ Plaque and Tau Hyperphosphorylation in a Mouse Model of Alzheimer's Disease.Front Mol Neurosci. 2019 Aug 16;12:192. doi: 10.3389/fnmol.2019.00192. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31474828 Free PMC article.
-
The schizophrenia risk gene Map2k7 regulates responding in a novel contingency-shifting rodent touchscreen gambling task.Dis Model Mech. 2022 Mar 1;15(3):dmm049310. doi: 10.1242/dmm.049310. Epub 2022 Mar 11. Dis Model Mech. 2022. PMID: 35275161 Free PMC article.
References
-
- Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, Sweatt JD, Li WP, Adelmann G, Frotscher M, Hammer RE, Herz J (2005) Modulation of synaptic plasticity and memory by reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron 47:567–579. 10.1016/j.neuron.2005.07.007 - DOI - PubMed
-
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A (2010) Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci 30:4590–4600. 10.1523/JNEUROSCI.0640-10.2010 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
