A novel assay for the introduction of the vinyl ether double bond into plasmalogens using pyrene-labeled substrates
- PMID: 29540573
- PMCID: PMC5928432
- DOI: 10.1194/jlr.D080283
A novel assay for the introduction of the vinyl ether double bond into plasmalogens using pyrene-labeled substrates
Abstract
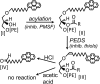
Plasmanylethanolamine desaturase (PEDS) (EC 1.14.99.19) introduces the 1-prime double bond into plasmalogens, one of the most abundant phospholipids in the human body. This labile membrane enzyme has not been purified and its coding sequence is unknown. Previous assays for this enzyme used radiolabeled substrates followed by multistep processing. We describe here a straight-forward method for the quantification of PEDS in enzyme incubation mixtures using pyrene-labeled substrates and reversed-phase HPLC with fluorescence detection. After stopping the reaction with hydrochloric acid in acetonitrile, the mixture was directly injected into the HPLC system without the need of lipid extraction. The substrate, 1-O-pyrenedecyl-2-acyl-sn-glycero-3-phosphoethanolamine, and the lyso-substrate, 1-O-pyrenedecyl-sn-glycero-3-phosphoethanolamine, were prepared from RAW-12 cells deficient in PEDS activity and were compared for their performance in the assay. Plasmalogen levels in mouse tissues and in cultured cells did not correlate with PEDS levels, indicating that the desaturase might not be the rate limiting step for plasmalogen biosynthesis. Among selected mouse organs, the highest activities were found in kidney and in spleen. Incubation of intact cultivated mammalian cells with 1-O-pyrenedecyl-sn-glycerol, extraction of lipids, and treatment with hydrochloric or acetic acid in acetonitrile allowed sensitive monitoring of PEDS activity in intact cells.
Keywords: ether lipid; lyso-phosphatidyl ethanolamine; phosphatidyl ethanolamine; plasmanylethanolamine desaturase.
Copyright © 2018 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
The TMEM189 gene encodes plasmanylethanolamine desaturase which introduces the characteristic vinyl ether double bond into plasmalogens.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7792-7798. doi: 10.1073/pnas.1917461117. Epub 2020 Mar 24. Proc Natl Acad Sci U S A. 2020. PMID: 32209662 Free PMC article.
-
Identification of a lysophospholipase C that may be responsible for the biosynthesis of choline plasmalogens by Madin-Darby canine kidney cells.J Biol Chem. 1993 Dec 5;268(34):25500-8. J Biol Chem. 1993. PMID: 8244986
-
A bacterial light response reveals an orphan desaturase for human plasmalogen synthesis.Science. 2019 Oct 4;366(6461):128-132. doi: 10.1126/science.aay1436. Science. 2019. PMID: 31604315
-
Normal plasmalogen levels are maintained in tissues from mice with hepatocyte-specific deletion in peroxin 5.Brain Res Bull. 2023 Feb;193:158-165. doi: 10.1016/j.brainresbull.2022.12.007. Epub 2022 Dec 28. Brain Res Bull. 2023. PMID: 36584717 Review.
-
Plasmalogens and Photooxidative Stress Signaling in Myxobacteria, and How it Unmasked CarF/TMEM189 as the Δ1'-Desaturase PEDS1 for Human Plasmalogen Biosynthesis.Front Cell Dev Biol. 2022 May 11;10:884689. doi: 10.3389/fcell.2022.884689. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646900 Free PMC article. Review.
Cited by
-
Lipidomics analysis reveals new insights into crisp grass carp associated with meat texture.Heliyon. 2024 May 31;10(11):e32179. doi: 10.1016/j.heliyon.2024.e32179. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38868033 Free PMC article.
-
Functional characterization of TMEM86A and TMEM86B mutants by a novel lysoplasmalogenase assay.J Lipid Res. 2025 Apr;66(4):100766. doi: 10.1016/j.jlr.2025.100766. Epub 2025 Feb 28. J Lipid Res. 2025. PMID: 40024572 Free PMC article.
-
Endothelial Slc35a1 Deficiency Causes Loss of LSEC Identity and Exacerbates Neonatal Lipid Deposition in the Liver in Mice.Cell Mol Gastroenterol Hepatol. 2024;17(6):1039-1061. doi: 10.1016/j.jcmgh.2024.03.002. Epub 2024 Mar 11. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38467191 Free PMC article.
-
Lipidomics Analysis Explores the Mechanism of Renal Injury in Rat Induced by 3-MCPD.Toxics. 2023 May 25;11(6):479. doi: 10.3390/toxics11060479. Toxics. 2023. PMID: 37368578 Free PMC article.
-
The TMEM189 gene encodes plasmanylethanolamine desaturase which introduces the characteristic vinyl ether double bond into plasmalogens.Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7792-7798. doi: 10.1073/pnas.1917461117. Epub 2020 Mar 24. Proc Natl Acad Sci U S A. 2020. PMID: 32209662 Free PMC article.
References
-
- Nagan N., and Zoeller R. A.. 2001. Plasmalogens: biosynthesis and functions. Prog. Lipid Res. 40: 199–229. - PubMed
-
- Braverman N. E., and Moser A. B.. 2012. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta. 1822: 1442–1452. - PubMed
-
- Paltauf F. 1972. Plasmalogen biosynthesis in a cell-free system. Enzymic desaturation of 1-O-alkyl (2-acyl) glycerophosphoryl ethanolamine. FEBS Lett. 20: 79–82. - PubMed
-
- Wykle R. L., Blank M. L., Malone B., and Snyder F.. 1972. Evidence for a mixed function oxidase in the biosynthesis of ethanolamine plasmalogens from 1-alkyl-2-acyl-sn-glycero-3-phosphorylethanolamine. J. Biol. Chem. 247: 5442–5447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

