Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome
- PMID: 29540584
- PMCID: PMC5890607
- DOI: 10.1212/WNL.0000000000005254
Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome
Abstract
Objective: To evaluate the safety and preliminary pharmacokinetics of a pharmaceutical formulation of purified cannabidiol (CBD) in children with Dravet syndrome.
Methods: Patients aged 4-10 years were randomized 4:1 to CBD (5, 10, or 20 mg/kg/d) or placebo taken twice daily. The double-blind trial comprised 4-week baseline, 3-week treatment (including titration), 10-day taper, and 4-week follow-up periods. Completers could continue in an open-label extension. Multiple pharmacokinetic blood samples were taken on the first day of dosing and at end of treatment for measurement of CBD, its metabolites 6-OH-CBD, 7-OH-CBD, and 7-COOH-CBD, and antiepileptic drugs (AEDs; clobazam and metabolite N-desmethylclobazam [N-CLB], valproate, levetiracetam, topiramate, and stiripentol). Safety assessments were clinical laboratory tests, physical examinations, vital signs, ECGs, adverse events (AEs), seizure frequency, and suicidality.
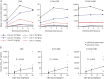
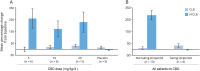
Results: Thirty-four patients were randomized (10, 8, and 9 to the 5, 10, and 20 mg/kg/d CBD groups, and 7 to placebo); 32 (94%) completed treatment. Exposure to CBD and its metabolites was dose-proportional (AUC0-t). CBD did not affect concomitant AED levels, apart from an increase in N-CLB (except in patients taking stiripentol). The most common AEs on CBD were pyrexia, somnolence, decreased appetite, sedation, vomiting, ataxia, and abnormal behavior. Six patients taking CBD and valproate developed elevated transaminases; none met criteria for drug-induced liver injury and all recovered. No other clinically relevant safety signals were observed.
Conclusions: Exposure to CBD and its metabolites increased proportionally with dose. An interaction with N-CLB was observed, likely related to CBD inhibition of cytochrome P450 subtype 2C19. CBD resulted in more AEs than placebo but was generally well-tolerated.
Classification of evidence: This study provides Class I evidence that for children with Dravet syndrome, CBD resulted in more AEs than placebo but was generally well-tolerated.
Copyright © 2018 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

References
-
- Devinsky O, Cross JH, Laux L, et al. . Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017;376:2011–2020. - PubMed
-
- O'Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav 2017;70:341–348. - PubMed
-
- Devinsky O, Marsh E, Friedman D, et al. . Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol 2016;15:270–278. - PubMed
-
- Gofshteyn JS, Wilfong A, Devinsky O, et al. . Cannabidiol as a potential treatment for febrile infection-related epilepsy syndrome (FIRES) in the acute and chronic phases. J Child Neurol 2017;32:35–40. - PubMed
-
- Hess EJ, Moody KA, Geffrey AL, et al. . Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 2016;57:1617–1624. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
