Exacerbated Apoptosis of Cells Infected with Infectious Bursal Disease Virus upon Exposure to Interferon Alpha
- PMID: 29540594
- PMCID: PMC5952143
- DOI: 10.1128/JVI.00364-18
Exacerbated Apoptosis of Cells Infected with Infectious Bursal Disease Virus upon Exposure to Interferon Alpha
Abstract
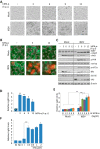
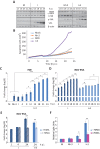
Infectious bursal disease virus (IBDV) belongs to the Birnaviridae family and is the etiological agent of a highly contagious and immunosuppressive disease (IBD) that affects domestic chickens (Gallus gallus). IBD or Gumboro disease leads to high rates of morbidity and mortality of infected animals and is responsible for major economic losses to the poultry industry worldwide. IBD is characterized by a massive loss of IgM-bearing B lymphocytes and the destruction of the bursa of Fabricius. The molecular bases of IBDV pathogenicity are still poorly understood; nonetheless, an exacerbated cytokine immune response and B cell depletion due to apoptosis are considered main factors that contribute to the severity of the disease. Here we have studied the role of type I interferon (IFN) in IBDV infection. While IFN pretreatment confers protection against subsequent IBDV infection, the addition of IFN to infected cell cultures early after infection drives massive apoptotic cell death. Downregulation of double-stranded RNA (dsRNA)-dependent protein kinase (PKR), tumor necrosis factor alpha (TNF-α), or nuclear factor κB (NF-κB) expression drastically reduces the extent of apoptosis, indicating that they are critical proteins in the apoptotic response induced by IBDV upon treatment with IFN-α. Our results indicate that IBDV genomic dsRNA is a major viral factor that contributes to the triggering of apoptosis. These findings provide novel insights into the potential mechanisms of IBDV-induced immunosuppression and pathogenesis in chickens.IMPORTANCE IBDV infection represents an important threat to the poultry industry worldwide. IBDV-infected chickens develop severe immunosuppression, which renders them highly susceptible to secondary infections and unresponsive to vaccination against other pathogens. The early dysregulation of the innate immune response led by IBDV infection and the exacerbated apoptosis of B cells have been proposed as the main factors that contribute to virus-induced immunopathogenesis. Our work contributes for the first time to elucidating a potential mechanism driving the apoptotic death of IBDV-infected cells upon exposure to type I IFN. We provide solid evidence about the critical importance of PKR, TNF-α, and NF-κB in this phenomenon. The described mechanism could facilitate the early clearance of infected cells, thereby aiding in the amelioration of IBDV-induced pathogenesis, but it could also contribute to B cell depletion and immunosuppression. The balance between these two opposing effects might be dramatically affected by the genetic backgrounds of both the host and the infecting virus strain.
Keywords: apoptosis; infectious bursal disease virus; interferon; pathogenesis.
Copyright © 2018 American Society for Microbiology.
Figures

Similar articles
-
Modified live infectious bursal disease virus (IBDV) vaccine delays infection of neonatal broiler chickens with variant IBDV compared to turkey herpesvirus (HVT)-IBDV vectored vaccine.Vaccine. 2017 Feb 7;35(6):882-888. doi: 10.1016/j.vaccine.2017.01.005. Epub 2017 Jan 12. Vaccine. 2017. PMID: 28089549
-
Differences in genetic background influence the induction of innate and acquired immune responses in chickens depending on the virulence of the infecting infectious bursal disease virus (IBDV) strain.Vet Immunol Immunopathol. 2010 May 15;135(1-2):79-92. doi: 10.1016/j.vetimm.2009.11.005. Epub 2009 Nov 18. Vet Immunol Immunopathol. 2010. PMID: 20005576
-
Infectious Bursal Disease: a complex host-pathogen interaction.Dev Comp Immunol. 2013 Nov;41(3):429-38. doi: 10.1016/j.dci.2013.03.017. Epub 2013 Apr 6. Dev Comp Immunol. 2013. PMID: 23567344 Review.
-
Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery.Arch Virol. 2002;147(2):285-304. doi: 10.1007/s705-002-8320-2. Arch Virol. 2002. PMID: 11890524
-
Infectious bursal disease virus of chickens: pathogenesis and immunosuppression.Dev Comp Immunol. 2000 Mar-Apr;24(2-3):223-35. doi: 10.1016/s0145-305x(99)00074-9. Dev Comp Immunol. 2000. PMID: 10717289 Review.
Cited by
-
Ex vivo rescue of recombinant very virulent IBDV using a RNA polymerase II driven system and primary chicken bursal cells.Sci Rep. 2020 Aug 6;10(1):13298. doi: 10.1038/s41598-020-70095-x. Sci Rep. 2020. PMID: 32764663 Free PMC article.
-
Characterization and pathogenicity of a novel variant infectious bursal disease virus in China.Front Microbiol. 2023 Mar 17;13:1039259. doi: 10.3389/fmicb.2022.1039259. eCollection 2022. Front Microbiol. 2023. PMID: 37008302 Free PMC article.
-
Type I Interferon acts as a major barrier to the establishment of infectious bursal disease virus (IBDV) persistent infections.J Virol. 2021 Mar 1;95(5):e02017-20. doi: 10.1128/JVI.02017-20. Epub 2020 Dec 16. J Virol. 2021. PMID: 33328313 Free PMC article.
-
Interaction between chicken TRIM25 and MDA5 and their role in mediated antiviral activity against IBDV infection.Front Microbiol. 2022 Nov 28;13:1068328. doi: 10.3389/fmicb.2022.1068328. eCollection 2022. Front Microbiol. 2022. PMID: 36519174 Free PMC article.
-
Genetic resilience in chickens against bacterial, viral and protozoal pathogens.Front Vet Sci. 2022 Nov 10;9:1032983. doi: 10.3389/fvets.2022.1032983. eCollection 2022. Front Vet Sci. 2022. PMID: 36439341 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

