Physical Exercise Reduces Cytotoxicity and Up-Regulates Nrf2 and UPR Expression in Circulating Cells of Peripheral Artery Disease Patients: An Hypoxic Adaptation?
- PMID: 29540636
- PMCID: PMC6143780
- DOI: 10.5551/jat.42432
Physical Exercise Reduces Cytotoxicity and Up-Regulates Nrf2 and UPR Expression in Circulating Cells of Peripheral Artery Disease Patients: An Hypoxic Adaptation?
Abstract
Aim: Ischemia-reperfusion (I-R) produces reactive oxygen species (ROS) that damage cells and favour cytotoxicity and apoptosis in peripheral artery disease (PAD) patients. Since brief episodes of I-R (ischemic conditioning) protect cells against ischemic harms, we evaluated whether a short-course of supervised treadmill training, characterized by repeated episodes of I-R, makes peripheral blood mononuclear cells (PBMCs) from PAD patients with intermittent claudication more resistant to I-R injuries by reducing oxidative stress and by inducing an adaptative response of unfolded protein response (UPR) and nuclear factor-E2-related factor (Nrf2) pathway expression.
Methods: 24 PAD patients underwent 21 sessions of treadmill training and a treadmill test as indicator of acute response to I-R.
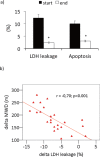
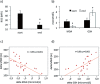
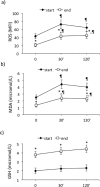
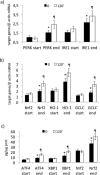
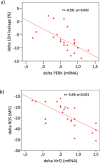
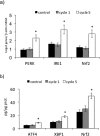
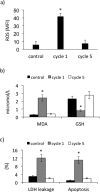
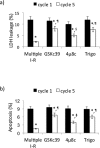
Results: Maximal and pain free walking distance improved (p<0.01), whereas LDH leakage and apoptosis of PBMCs decreased (p<0.01); plasma malondialdehyde and ROS generation in PBMCs declined, while plasma glutathione augmented (p<0.01). Moreover we demonstrated an up-regulation of UPR and Nrf2 expression in PBMCs (p<0.01). To understand whether treadmill training may act as a trigger of ischemic conditioning, we examined the effect of repeated episodes of I-R on adaptative response in PBMCs derived from the patients. We showed an up-regulation of UPR and Nrf2 gene expression (p<0.01), while oxidative stress and cytotoxicity, after an initial increase, declined (p<0.01). This positive effect on cytotoxicity was reduced after inhibition of UPR and Nrf2 pathways.
Conclusions: Treadmill training in PAD patients through UPR and Nrf2 up-regulation may trigger hypoxic adaptation similar to conditioning, thus modifying cell survival.
Keywords: Nrf2; Oxidative stress; PAD; UPR.
Conflict of interest statement
All authors declare that they have no conflict of interest or financial disclosures.
Figures
Similar articles
-
Increased endoplasmic reticulum stress and Nrf2 repression in peripheral blood mononuclear cells of patients with stable coronary artery disease.Free Radic Biol Med. 2014 Mar;68:178-85. doi: 10.1016/j.freeradbiomed.2013.12.017. Epub 2013 Dec 27. Free Radic Biol Med. 2014. PMID: 24373961
-
Nrf2 expression is increased in peripheral blood mononuclear cells derived from mild-moderate ex-smoker COPD patients with persistent oxidative stress.Int J Chron Obstruct Pulmon Dis. 2016 Jul 28;11:1733-43. doi: 10.2147/COPD.S102218. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27555763 Free PMC article.
-
Endoplasmic reticulum stress and Nrf2 repression in circulating cells of type 2 diabetic patients without the recommended glycemic goals.Free Radic Res. 2015 Mar;49(3):244-52. doi: 10.3109/10715762.2014.997229. Epub 2015 Feb 6. Free Radic Res. 2015. PMID: 25511473
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
Endoplasmic reticulum stress and Nrf2 signaling in cardiovascular diseases.Free Radic Biol Med. 2015 Nov;88(Pt B):233-242. doi: 10.1016/j.freeradbiomed.2015.05.027. Epub 2015 Jun 4. Free Radic Biol Med. 2015. PMID: 26051167 Review.
Cited by
-
Harnessing the cardiovascular benefits of exercise: Are Nrf2 activators useful?Sports Med Health Sci. 2021 May 4;3(2):70-79. doi: 10.1016/j.smhs.2021.04.002. eCollection 2021 Jun. Sports Med Health Sci. 2021. PMID: 35782161 Free PMC article. Review.
-
Pathophysiology of Peripheral Arterial Disease (PAD): A Review on Oxidative Disorders.Int J Mol Sci. 2020 Jun 20;21(12):4393. doi: 10.3390/ijms21124393. Int J Mol Sci. 2020. PMID: 32575692 Free PMC article. Review.
-
Understanding the role of the cytoprotective transcription factor nuclear factor erythroid 2-related factor 2-lessons from evolution, the animal kingdom and rare progeroid syndromes.Nephrol Dial Transplant. 2020 Dec 4;35(12):2036-2045. doi: 10.1093/ndt/gfz120. Nephrol Dial Transplant. 2020. PMID: 31302696 Free PMC article. Review.
-
SOD2 mRNA as a potential biomarker for exercise: interventional and cross-sectional research in healthy subjects.J Clin Biochem Nutr. 2021 Sep;69(2):137-144. doi: 10.3164/jcbn.21-24. Epub 2021 May 8. J Clin Biochem Nutr. 2021. PMID: 34616105 Free PMC article.
-
Ezetimibe Prevents Ischemia/Reperfusion-Induced Oxidative Stress and Up-Regulates Nrf2/ARE and UPR Signaling Pathways.Antioxidants (Basel). 2020 Apr 23;9(4):349. doi: 10.3390/antiox9040349. Antioxidants (Basel). 2020. PMID: 32340270 Free PMC article.
References
-
- Gillani S, Cao J, Suzuki T, Hak DJ: The effect of ischemia reperfusion injury on skeletal muscle, Injury, 2012; 43: 670-675 - PubMed
-
- Pipinos II, Swanson SA, Zhu Z, Nella AA, Weiss DJ, Gutti TL, McComb RD, Baxter BT, Lynch TG, Casale GP: Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage, Am J Physiol Regul Integr Comp Physiol, 2008; 295: R290-R296 - PMC - PubMed
-
- Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, Baxter BT, Lynch TG, Dodd SL: Mitochondrial defects and oxidative damage in patients with peripheral arterial disease, Free Radic Biol Med, 2006; 41: 262-269 - PubMed
-
- Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, Filis KA, Sabbah HN: Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease, J Vasc Surg, 2003; 38: 827-832 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

