miR-381-3p knockdown improves intestinal epithelial proliferation and barrier function after intestinal ischemia/reperfusion injury by targeting nurr1
- PMID: 29540663
- PMCID: PMC5852084
- DOI: 10.1038/s41419-018-0450-z
miR-381-3p knockdown improves intestinal epithelial proliferation and barrier function after intestinal ischemia/reperfusion injury by targeting nurr1
Abstract
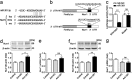
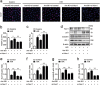
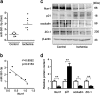
Impairment in gut barrier function induced by intestinal ischemia/reperfusion (I/R) injury is associated with high morbidity and mortality. Intestinal barrier function requires the tight coordination of epithelial migration, proliferation and differentiation. We previously observed that nuclear receptor-related protein 1 (nurr1)-mediated proliferative pathway was impaired in intestinal I/R injury. Here, we aimed to assess the effect of nurr1 on intestinal barrier function and to evaluate microRNA (miRNA)-nurr1-mediated restoration of intestinal barrier function in intestinal I/R injury. We induced an in vivo intestinal I/R injury mouse model by clamping and then releasing the superior mesenteric artery. We also performed an in vitro study in which we exposed Caco-2 and IEC-6 cells to hypoxia/reoxygenation (H/R) conditions to stimulate intestinal I/R injury. Our results demonstrated that nurr1 regulated intestinal epithelial development and barrier function after intestinal I/R injury. miR-381-3p, which directly suppressed nurr1 translation, was identified by microarray and bioinformatics analysis. miR-381-3p inhibition enhanced intestinal epithelial proliferation and barrier function in vitro and in vivo and also attenuated remote organ injury and improved survival. Importantly, nurr1 played an indispensable role in the protective effect of miR-381-3p inhibition. Collectively, these findings show that miR-381-3p inhibition mitigates intestinal I/R injury by enhancing nurr1-mediated intestinal epithelial proliferation and barrier function. This discovery may lead to the development of therapeutic interventions for intestinal I/R injury.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




Similar articles
-
MicroRNA files in the prevention of intestinal ischemia/reperfusion injury by hydrogen rich saline.Biosci Rep. 2020 Jan 31;40(1):BSR20191043. doi: 10.1042/BSR20191043. Biosci Rep. 2020. PMID: 31789347 Free PMC article.
-
Nurr1 promotes intestinal regeneration after ischemia/reperfusion injury by inhibiting the expression of p21 (Waf1/Cip1).J Mol Med (Berl). 2017 Jan;95(1):83-95. doi: 10.1007/s00109-016-1464-6. Epub 2016 Aug 24. J Mol Med (Berl). 2017. PMID: 27553040
-
Inhibition of miR-142-3p promotes intestinal epithelial proliferation and barrier function after ischemia/reperfusion injury by targeting FoxM1.Mol Cell Biochem. 2025 Feb;480(2):1121-1135. doi: 10.1007/s11010-024-05038-5. Epub 2024 May 31. Mol Cell Biochem. 2025. PMID: 38819598
-
Emerging roles of microRNAs in intestinal ischemia/reperfusion-induced injury: a review.J Physiol Biochem. 2020 Nov;76(4):525-537. doi: 10.1007/s13105-020-00772-y. Epub 2020 Nov 3. J Physiol Biochem. 2020. PMID: 33140255 Review.
-
Unveiling the molecular complexity of intestinal ischemia-reperfusion injury through omics technologies.Proteomics. 2024 Jun;24(12-13):e2300160. doi: 10.1002/pmic.202300160. Epub 2024 Mar 13. Proteomics. 2024. PMID: 38477684 Review.
Cited by
-
miR-138-5p ameliorates intestinal barrier disruption caused by acute superior mesenteric vein thrombosis injury by inhibiting the NLRP3/HMGB1 axis.PeerJ. 2024 Feb 21;12:e16692. doi: 10.7717/peerj.16692. eCollection 2024. PeerJ. 2024. PMID: 38406274 Free PMC article.
-
MiR-1-3p and MiR-124-3p Synergistically Damage the Intestinal Barrier in the Ageing Colon.J Crohns Colitis. 2022 May 10;16(4):656-667. doi: 10.1093/ecco-jcc/jjab179. J Crohns Colitis. 2022. PMID: 34628497 Free PMC article.
-
Intestinal ischemia-reperfusion induces the release of IL-17A to regulate cell inflammation, apoptosis and barrier damage.Exp Ther Med. 2022 Feb;23(2):158. doi: 10.3892/etm.2021.11081. Epub 2021 Dec 17. Exp Ther Med. 2022. PMID: 35069839 Free PMC article.
-
NR4A2 expression is not altered in placentas from cases of growth restriction or preeclampsia, but is reduced in hypoxic cytotrophoblast.Sci Rep. 2021 Oct 19;11(1):20670. doi: 10.1038/s41598-021-00192-y. Sci Rep. 2021. PMID: 34667209 Free PMC article.
-
MicroRNA files in the prevention of intestinal ischemia/reperfusion injury by hydrogen rich saline.Biosci Rep. 2020 Jan 31;40(1):BSR20191043. doi: 10.1042/BSR20191043. Biosci Rep. 2020. PMID: 31789347 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

