The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway
- PMID: 29540694
- PMCID: PMC5851994
- DOI: 10.1038/s41419-018-0436-x
The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway
Abstract
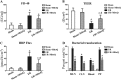
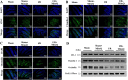
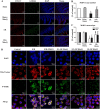
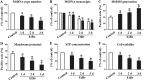
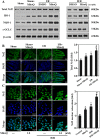
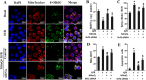
Disruption of the mucosal barrier following intestinal ischemia reperfusion (I/R) is life threatening in clinical practice. Mitochondrial dysfunction and oxidative stress significantly contribute to the early phase of I/R injury and amplify the inflammatory response. MitoQ is a mitochondrially targeted antioxidant that exerts protective effects following I/R injury. In the present study, we aimed to determine whether and how MitoQ protects intestinal epithelial cells (IECs) from I/R injury. In both in vivo and in vitro studies, we found that MitoQ pretreatment downregulated I/R-induced oxidative stress and stabilized the intestinal barrier, as evidenced by MitoQ-treated I/R mice exhibiting attenuated intestinal hyperpermeability, inflammatory response, epithelial apoptosis, and tight junction damage compared to controls. Mechanistically, I/R elevated mitochondrial 8-hydroxyguanine content, reduced mitochondrial DNA (mtDNA) copy number and mRNA transcription levels, and induced mitochondrial disruption in IECs. However, MitoQ pretreatment dramatically inhibited these deleterious effects. mtDNA depletion alone was sufficient to induce apoptosis and mitochondrial dysfunction of IECs. Mitochondrial transcription factor A (TFAM), a key activator of mitochondrial transcription, was significantly reduced during I/R injury, a phenomenon that was prevented by MitoQ treatment. Furthermore, we observed that thee protective properties of MitoQ were affected by upregulation of cellular antioxidant genes, including HO-1, NQO-1, and γ-GCLC. Transfection with Nrf2 siRNA in IECs exposed to hypoxia/reperfusion conditions partially blocked the effects of MitoQ on mtDNA damage and mitochondrial oxidative stress. In conclusion, our data suggest that MitoQ exerts protective effect on I/R-induced intestinal barrier dysfunction.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
MitoQ Modulates Lipopolysaccharide-Induced Intestinal Barrier Dysfunction via Regulating Nrf2 Signaling.Mediators Inflamm. 2020 Apr 11;2020:3276148. doi: 10.1155/2020/3276148. eCollection 2020. Mediators Inflamm. 2020. PMID: 32351320 Free PMC article.
-
The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1.Redox Biol. 2017 Apr;11:297-311. doi: 10.1016/j.redox.2016.12.022. Epub 2016 Dec 21. Redox Biol. 2017. PMID: 28033563 Free PMC article.
-
MitoQ protects against high glucose-induced brain microvascular endothelial cells injury via the Nrf2/HO-1 pathway.J Pharmacol Sci. 2021 Jan;145(1):105-114. doi: 10.1016/j.jphs.2020.10.007. Epub 2020 Oct 30. J Pharmacol Sci. 2021. PMID: 33357768
-
MitoQ--a mitochondria-targeted antioxidant.IDrugs. 2007 Jun;10(6):399-412. IDrugs. 2007. PMID: 17642004 Review.
-
Mitochondria-Targeted Drugs.Curr Mol Pharmacol. 2019;12(3):202-214. doi: 10.2174/1874467212666181127151059. Curr Mol Pharmacol. 2019. PMID: 30479224 Free PMC article. Review.
Cited by
-
Pharmacological Protection against Ischemia-Reperfusion Injury by Regulating the Nrf2-Keap1-ARE Signaling Pathway.Antioxidants (Basel). 2021 May 21;10(6):823. doi: 10.3390/antiox10060823. Antioxidants (Basel). 2021. PMID: 34063933 Free PMC article. Review.
-
Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium.Cells. 2022 Oct 31;11(21):3441. doi: 10.3390/cells11213441. Cells. 2022. PMID: 36359839 Free PMC article.
-
Oxidative stress in intervertebral disc degeneration: Molecular mechanisms, pathogenesis and treatment.Cell Prolif. 2023 Sep;56(9):e13448. doi: 10.1111/cpr.13448. Epub 2023 Mar 13. Cell Prolif. 2023. PMID: 36915968 Free PMC article. Review.
-
Mitochondrial Dysfunction in Cardiovascular Diseases: Potential Targets for Treatment.Front Cell Dev Biol. 2022 May 13;10:841523. doi: 10.3389/fcell.2022.841523. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35646910 Free PMC article. Review.
-
Mitochondrial Management of Reactive Oxygen Species.Antioxidants (Basel). 2021 Nov 17;10(11):1824. doi: 10.3390/antiox10111824. Antioxidants (Basel). 2021. PMID: 34829696 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

