Porous 3D Prussian blue/cellulose aerogel as a decorporation agent for removal of ingested cesium from the gastrointestinal tract
- PMID: 29540724
- PMCID: PMC5851989
- DOI: 10.1038/s41598-018-22715-w
Porous 3D Prussian blue/cellulose aerogel as a decorporation agent for removal of ingested cesium from the gastrointestinal tract
Abstract
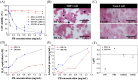
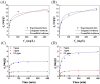
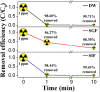
In the present study, we successfully synthesized a porous three-dimensional Prussian blue-cellulose aerogel (PB-CA) composite and used it as a decorporation agent for the selective removal of ingested cesium ions (Cs+) from the gastrointestinal (GI) tract. The safety of the PB-CA composite was evaluated through an in vitro cytotoxicity study using macrophage-like THP-1 cells and Caco-2 intestinal epithelial cells. The results revealed that the PB-CA composite was not cytotoxic. An adsorption study to examine the efficiency of the decorporation agent was conducted using a simulated intestinal fluid (SIF). The adsorption isotherm was fitted to the Langmuir model with a maximum Cs+ adsorption capacity of 13.70 mg/g in SIF that followed pseudo-second-order kinetics. The PB-CA composite showed excellent stability in SIF with a maximum Cs+ removal efficiency of 99.43%. The promising safety toxicology profile, remarkable Cs+ adsorption efficacy, and excellent stability of the composite demonstrated its great potential for use as an orally administered drug for the decorporation of Cs+ from the GI tract.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Awual MR, et al. Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents. Chem. Eng. J. 2014;242:127–135. doi: 10.1016/j.cej.2013.12.072. - DOI
-
- Kumamoto Y, et al. Meridional distribution of Fukushima-derived radiocesium in surface seawater along a trans-Pacific line from the Arctic to Antarctic Oceans in summer 2012. J. Radioanal. Nucl. Chem. 2016;307:1703–1710. doi: 10.1007/s10967-015-4439-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

