The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response
- PMID: 29540995
- PMCID: PMC5818902
- DOI: 10.1155/2018/5378284
The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response
Abstract
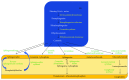
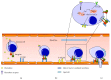
This review provides an overview on components of the sphingolipid superfamily, on their localization and metabolism. Information about the sphingolipid biological activity in cell physiopathology is given. Recent studies highlight the role of sphingolipids in inflammatory process. We summarize the emerging data that support the different roles of the sphingolipid members in specific phases of inflammation: (1) migration of immune cells, (2) recognition of exogenous agents, and (3) activation/differentiation of immune cells.
Figures
Similar articles
-
Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation.Nutrients. 2017 Oct 28;9(11):1180. doi: 10.3390/nu9111180. Nutrients. 2017. PMID: 29143791 Free PMC article. Review.
-
Inflammatory response and its relation to sphingolipid metabolism proteins: Chaperones as potential indirect anti-inflammatory agents.Adv Protein Chem Struct Biol. 2019;114:153-219. doi: 10.1016/bs.apcsb.2018.09.004. Epub 2018 Nov 28. Adv Protein Chem Struct Biol. 2019. PMID: 30635081 Review.
-
The role of sphingolipids in drug metabolism and transport.Expert Opin Drug Metab Toxicol. 2013 Mar;9(3):319-31. doi: 10.1517/17425255.2013.748749. Epub 2013 Jan 7. Expert Opin Drug Metab Toxicol. 2013. PMID: 23289866 Review.
-
Molecular facets of sphingolipids: mediators of diseases.Biotechnol J. 2009 Jul;4(7):1028-41. doi: 10.1002/biot.200800322. Biotechnol J. 2009. PMID: 19579220 Review.
-
Fostering Inflammatory Bowel Disease: Sphingolipid Strategies to Join Forces.Mediators Inflamm. 2016;2016:3827684. doi: 10.1155/2016/3827684. Epub 2016 Jan 5. Mediators Inflamm. 2016. PMID: 26880864 Free PMC article. Review.
Cited by
-
Ceramide releases exosomes with a specific miRNA signature for cell differentiation.Sci Rep. 2023 Jul 7;13(1):10993. doi: 10.1038/s41598-023-38011-1. Sci Rep. 2023. PMID: 37419964 Free PMC article.
-
Frontloading of stress response genes enhances robustness to environmental change in chimeric corals.BMC Biol. 2022 Jul 26;20(1):167. doi: 10.1186/s12915-022-01371-7. BMC Biol. 2022. PMID: 35879753 Free PMC article.
-
Steryl Glycosides in Fungal Pathogenesis: An Understudied Immunomodulatory Adjuvant.J Fungi (Basel). 2020 Feb 24;6(1):25. doi: 10.3390/jof6010025. J Fungi (Basel). 2020. PMID: 32102324 Free PMC article. Review.
-
Metabolomics analysis of a mouse model for chronic exposure to ambient PM2.5.Environ Pollut. 2019 Apr;247:953-963. doi: 10.1016/j.envpol.2019.01.118. Epub 2019 Feb 1. Environ Pollut. 2019. PMID: 30823350 Free PMC article.
-
Antifungal Drug Development: Targeting the Fungal Sphingolipid Pathway.J Fungi (Basel). 2020 Aug 20;6(3):142. doi: 10.3390/jof6030142. J Fungi (Basel). 2020. PMID: 32825250 Free PMC article. Review.
References
-
- Kolesnick R. N., Hemer M. R. Characterization of a ceramide kinase activity from human leukemia (HL-60) cells. Separation from diacylglycerol kinase activity. The Journal of Biological Chemistry. 1990;265(31):18803–18808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

