Depletion of Regulatory T Cells in Visceral Adipose Tissues Contributes to Insulin Resistance in Hashimoto's Thyroiditis
- PMID: 29541033
- PMCID: PMC5835527
- DOI: 10.3389/fphys.2018.00136
Depletion of Regulatory T Cells in Visceral Adipose Tissues Contributes to Insulin Resistance in Hashimoto's Thyroiditis
Abstract
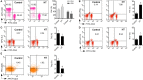
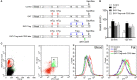
Hashimoto's Thyroiditis (HT) is a common organ-specific autoimmune disorder associated with a high incidence, and insulin resistance is highly related to autoimmune. Here, we examined the insulin sensitivity in HT patients and found decreased insulin sensitivity occurred in HT patients. To explore the relationship between impaired insulin sensitivity and immune status, we established HT model mice which showed similar pathological features and immune features to HT patients. In HT model mice, reinfusion of regulatory T cells (Tregs) from peripheral blood of normal mice could improve insulin sensitivity and decrease the inflammation. Anti-CD25 antibodies blocked beneficial effects from reinfusion of Tregs, but delayed administration of anti-CD25 antibodies could not abolished the effect from Tregs. Delayed administration of anti-CD25 antibodies abolished exogenous Tregs in peripheral blood, but there were increased exogenous Tregs located to visceral adipose tissues (VATs) which modulated the expression of cytokines in VATs. These findings suggest that insulin resistance exists in HT patients and it associates with the decreased Tregs and increased inflammation in the VATs.
Keywords: Hashimoto's Thyroiditis; cytokines; insulin resistance; regulatory T cells; visceral adipose tissues.
Figures





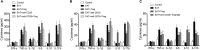
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

