A role for GPI-CD59 in promoting T-cell signal transduction via LAT
- PMID: 29541246
- PMCID: PMC5835848
- DOI: 10.3892/ol.2018.7908
A role for GPI-CD59 in promoting T-cell signal transduction via LAT
Abstract
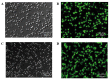
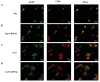
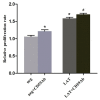
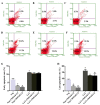
Cluster of differentiation 59 (CD59) is a glycosylphosphatidylinositol-anchored protein. Cross-linking of CD59 with specific monoclonal antibodies can cause a series of intracellular signal transduction events. However, the underlying molecular mechanisms are poorly understood. Linker for activation of T-cells (LAT) is a crucial adaptor protein in T-cell signaling, and its phosphorylation and palmitoylation are essential for its localization and function. In a previous study by the present authors, it was demonstrated that CD59 may be responsible for LAT palmitoylation, thereby regulating T-cell signal transduction. The present study detected the co-localization of LAT and CD59 in lipid rafts by transfecting Jurkat cells with lentivirus vectors carrying the LAT-enhanced green fluorescent protein fusion protein. In addition, LAT and CD59 were shown to have a synergistic effect on the proliferation of Jurkat cells. The results also indicated that CD59 may transfer the palmitate group from phosphatidylinositol to LAT to form LAT palmitate, which then localizes to lipid rafts to regulate T-cell activation. The results of the present study provided novel insights into the role of CD59 in T-cell signal transduction.
Keywords: T-cell; cluster of differentiation 59; glycosylphosphatidylinositol; linker for activation of T-cells; signal transduction.
Figures

Similar articles
-
[Mutation of palmitoylation site of linker for activation of T cells inhibits signal transduction mediated by glycosyl phosphatidyl inositol-anchored CD59 in T cells].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015 Aug;31(8):1013-6, 1021. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015. PMID: 26271970 Chinese.
-
[Impact of the palmitoylation of linker for activation of T cells on signal transduction pathway of CD59 in T cells].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013 Nov;29(11):1121-4. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013. PMID: 24200054 Chinese.
-
The glycosylphosphatidylinositol-anchored CD59 protein stimulates both T cell receptor zeta/ZAP-70-dependent and -independent signaling pathways in T cells.Eur J Immunol. 1995 Jul;25(7):1815-22. doi: 10.1002/eji.1830250704. Eur J Immunol. 1995. PMID: 7542590
-
LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways.Sci STKE. 2000 Dec 19;2000(63):re1. doi: 10.1126/stke.2000.63.re1. Sci STKE. 2000. PMID: 11752630 Review.
-
Alternative roles for CD59.Mol Immunol. 2007 Jan;44(1-3):73-81. doi: 10.1016/j.molimm.2006.06.019. Epub 2006 Aug 1. Mol Immunol. 2007. PMID: 16884774 Review.
Cited by
-
Amplification of Stem Genes: New Potential Metastatic Makers in Patients with an Early Form of Breast Cancer.J Korean Med Sci. 2019 Dec 23;34(49):e312. doi: 10.3346/jkms.2019.34.e312. J Korean Med Sci. 2019. PMID: 31858753 Free PMC article.
-
High Expression of PIGC Predicts Unfavorable Survival in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2021 Apr 6;8:211-222. doi: 10.2147/JHC.S297601. eCollection 2021. J Hepatocell Carcinoma. 2021. PMID: 33854986 Free PMC article.
-
Deciphering CD59: Unveiling Its Role in Immune Microenvironment and Prognostic Significance.Cancers (Basel). 2024 Nov 1;16(21):3699. doi: 10.3390/cancers16213699. Cancers (Basel). 2024. PMID: 39518137 Free PMC article.
-
Multiple CD59 Polymorphisms in Chinese Patients with Mycobacterium tuberculosis Infection.J Immunol Res. 2023 Apr 2;2023:1216048. doi: 10.1155/2023/1216048. eCollection 2023. J Immunol Res. 2023. PMID: 37050931 Free PMC article.
-
Relating GPI-Anchored Ly6 Proteins uPAR and CD59 to Viral Infection.Viruses. 2019 Nov 14;11(11):1060. doi: 10.3390/v11111060. Viruses. 2019. PMID: 31739586 Free PMC article. Review.
References
-
- Davis LS, Patel SS, Atkinson JP, Lipsky PE. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988;141:2246–2252. - PubMed
-
- Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5′-nucleotidase (CD73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood T cells to proliferate. J Immunol. 1989;143:1815–1821. - PubMed
-
- Presky DH, Low MG, Shevach EM. Role of phosphatidylinositol-anchored proteins in T cell activation. J Immunol. 1990;144:860–868. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
