A c-di-GMP-Modulating Protein Regulates Swimming Motility of Burkholderia cenocepacia in Response to Arginine and Glutamate
- PMID: 29541628
- PMCID: PMC5835511
- DOI: 10.3389/fcimb.2018.00056
A c-di-GMP-Modulating Protein Regulates Swimming Motility of Burkholderia cenocepacia in Response to Arginine and Glutamate
Abstract
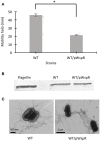
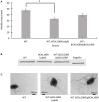
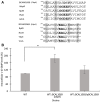
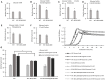
Burkholderia cenocepacia is an opportunistic bacterium that can thrive in different environments, including the amino acid-rich mucus of the cystic fibrosis (CF) lung. B. cenocepacia responds to the nutritional conditions that mimic the CF sputum by increasing flagellin expression and swimming motility. Individual amino acids also induce swimming but not flagellin expression. Here, we show that modulation of the second messenger cyclic dimeric guanosine monophosphate (c-di-GMP) levels by the PAS-containing c-di-GMP phosphodiesterase, BCAL1069 (CdpA), regulates the swimming motility of B. cenocepacia K56-2 in response to CF sputum nutritional conditions. Heterologous expression of WspR, a diguanylate cyclase, in B. cenocepacia K56-2 caused an increase in c-di-GMP levels and reduced swimming motility but did not affect flagellin expression or flagellar biosynthesis. After insertional mutagenesis of 12 putative genes encoding c-di-GMP metabolizing enzymes, one mutant of the locus BCAL1069 (cdpA), exhibited decreased swimming motility independent of flagellin expression in CF sputum nutritional conditions and an increase in intracellular c-di-GMP levels. The reduced swimming motility phenotype of the BCAL1069 mutant was observed in the presence of arginine and glutamate, but not of histidine, phenylalanine, or proline. The B. cenocepacia CdpA was also found to be involved in regulation of protease activity but not in biofilm formation. Altogether, these results highlight a role of B. cenocepacia BCAL1069 (CdpA) in sensing the nutritional conditions of the CF sputum and eliciting a pathogenic response that includes swimming motility toward amino acids and an increase in protease activity.
Keywords: BCAL1069; Burkholderia cenocepacia; Burkholderia cepacia complex; SCFM; c-di-GMP; cdpA; cystic fibrosis; motility.
Figures

Similar articles
-
Pellicle Biofilm Formation in Burkholderia cenocepacia J2315 is Epigenetically Regulated through WspH, a Hybrid Two-Component System Kinase-Response Regulator.J Bacteriol. 2022 May 17;204(5):e0001722. doi: 10.1128/jb.00017-22. Epub 2022 Apr 13. J Bacteriol. 2022. PMID: 35416687 Free PMC article.
-
Synthetic Cystic Fibrosis Sputum Medium Regulates Flagellar Biosynthesis through the flhF Gene in Burkholderia cenocepacia.Front Cell Infect Microbiol. 2016 Jun 14;6:65. doi: 10.3389/fcimb.2016.00065. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27379216 Free PMC article.
-
Thermoregulation of Biofilm Formation in Burkholderia pseudomallei Is Disrupted by Mutation of a Putative Diguanylate Cyclase.J Bacteriol. 2017 Feb 14;199(5):e00780-16. doi: 10.1128/JB.00780-16. Print 2017 Mar 1. J Bacteriol. 2017. PMID: 27956524 Free PMC article.
-
Targeting c-di-GMP Signaling, Biofilm Formation, and Bacterial Motility with Small Molecules.Methods Mol Biol. 2017;1657:419-430. doi: 10.1007/978-1-4939-7240-1_31. Methods Mol Biol. 2017. PMID: 28889311 Review.
-
Bacterial diguanylate cyclases: structure, function and mechanism in exopolysaccharide biofilm development.Biotechnol Adv. 2015 Jan-Feb;33(1):124-141. doi: 10.1016/j.biotechadv.2014.11.010. Epub 2014 Dec 10. Biotechnol Adv. 2015. PMID: 25499693 Review.
Cited by
-
Biofilm Signaling, Composition and Regulation in Burkholderia pseudomallei.J Microbiol Biotechnol. 2023 Jan 28;33(1):15-27. doi: 10.4014/jmb.2207.07032. Epub 2022 Oct 17. J Microbiol Biotechnol. 2023. PMID: 36451302 Free PMC article. Review.
-
Pellicle Biofilm Formation in Burkholderia cenocepacia J2315 is Epigenetically Regulated through WspH, a Hybrid Two-Component System Kinase-Response Regulator.J Bacteriol. 2022 May 17;204(5):e0001722. doi: 10.1128/jb.00017-22. Epub 2022 Apr 13. J Bacteriol. 2022. PMID: 35416687 Free PMC article.
-
Characterization and analysis of a novel diguanylate cyclase PA0847 from Pseudomonas aeruginosa PAO1.Infect Drug Resist. 2019 Mar 21;12:655-665. doi: 10.2147/IDR.S194462. eCollection 2019. Infect Drug Resist. 2019. PMID: 31114257 Free PMC article.
-
A c-di-GMP Signaling Cascade Controls Motility, Biofilm Formation, and Virulence in Burkholderia thailandensis.Appl Environ Microbiol. 2022 Apr 12;88(7):e0252921. doi: 10.1128/aem.02529-21. Epub 2022 Mar 24. Appl Environ Microbiol. 2022. PMID: 35323023 Free PMC article.
-
Correlation of extracellular polymeric substances and microbial community structure in denitrification biofilm exposed to adverse conditions.Microb Biotechnol. 2020 Nov;13(6):1889-1903. doi: 10.1111/1751-7915.13633. Epub 2020 Jul 23. Microb Biotechnol. 2020. PMID: 32700468 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

