Systematic Review of Health Economic Evaluation Studies Developed in Brazil from 1980 to 2013
- PMID: 29541630
- PMCID: PMC5835950
- DOI: 10.3389/fpubh.2018.00052
Systematic Review of Health Economic Evaluation Studies Developed in Brazil from 1980 to 2013
Abstract
Background: Brazil has sought to use economic evaluation to support healthcare decision-making processes. While a number of health economic evaluations (HEEs) have been conducted, no study has systematically reviewed the quality of Brazilian HEE. The objective of this systematic review was to provide an overview regarding the state of HEE research and to evaluate the number, characteristics, and quality of reporting of published HEE studies conducted in a Brazilian setting.
Methods: We systematically searched electronic databases (MEDLINE, EMBASE, Latin American, and Caribbean Literature on Health Sciences Database, Scientific Electronic Library Online, NHS Economic Evaluation Database, health technology assessment Database, Bireme, and Biblioteca Virtual em Saúde Economia da Saúde); citation indexes (SCOPUS, Web of Science), and Sistema de Informação da Rede Brasileira de Avaliação de Tecnologia em Saúde. Partial and full HEEs published between 1980 and 2013 that referred to a Brazilian setting were considered for inclusion.
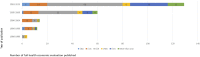
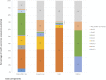
Results: In total, 535 studies were included in the review, 36.8% of these were considered to be full HEE. The category of healthcare technologies more frequently assessed were procedures (34.8%) and drugs (28.8%) which main objective was treatment (72.1%). Forty-four percent of the studies reported their funding source and 36% reported a conflict of interest. Overall, the full HEE quality of reporting was satisfactory. But some items were generally poorly reported and significant improvement is required: (1) methods used to estimate healthcare resource use quantities and unit costs, (2) methods used to estimate utility values, (3) sources of funding, and (4) conflicts of interest.
Conclusion: A steady number of HEE have been published in Brazil since 1980. To improve their contribution to inform national healthcare policy efforts need to be made to enhance the quality of reporting of HEEs and promote improvements in the way HEEs are designed, implemented (i.e., using sound methods for HEEs) and reported.
Keywords: Brazil; cost-benefit analysis; cost-effectiveness; economic evaluation; health technology assessment.
Figures




Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Health Economic Evaluations of Cancer in Brazil: A Systematic Review.Front Public Health. 2018 Jul 27;6:205. doi: 10.3389/fpubh.2018.00205. eCollection 2018. Front Public Health. 2018. PMID: 30101142 Free PMC article.
-
COVID-19 and zoonoses in Brazil: Environmental scan of one health preparedness and response.One Health. 2022 Jun;14:100400. doi: 10.1016/j.onehlt.2022.100400. Epub 2022 May 14. One Health. 2022. PMID: 35601224 Free PMC article.
-
Economic evaluations in gastroenterology in Brazil: A systematic review.World J Gastrointest Pharmacol Ther. 2016 Feb 6;7(1):162-70. doi: 10.4292/wjgpt.v7.i1.162. World J Gastrointest Pharmacol Ther. 2016. PMID: 26855823 Free PMC article.
-
A Systematic Review of Health Economic Evaluations and Budget Impact Analyses to Inform Healthcare Decision-Making in Central America.Appl Health Econ Health Policy. 2023 May;21(3):419-440. doi: 10.1007/s40258-023-00791-y. Epub 2023 Jan 31. Appl Health Econ Health Policy. 2023. PMID: 36720754
Cited by
-
Cost Effectiveness of Pharmacological Management for Osteoarthritis: A Systematic Review.Appl Health Econ Health Policy. 2022 May;20(3):351-370. doi: 10.1007/s40258-022-00717-0. Epub 2022 Feb 9. Appl Health Econ Health Policy. 2022. PMID: 35138600 Free PMC article.
-
A Systematic Review on Economic Evaluation Studies of Diagnostic and Therapeutic Interventions in the Middle East and North Africa.Appl Health Econ Health Policy. 2022 May;20(3):315-335. doi: 10.1007/s40258-021-00703-y. Epub 2021 Dec 21. Appl Health Econ Health Policy. 2022. PMID: 34931297
-
Economic evaluation of hepatitis A vaccines by income level of the country: A systematic review.Indian J Med Res. 2022 Sep;156(3):388-410. doi: 10.4103/ijmr.IJMR_1631_20. Indian J Med Res. 2022. PMID: 36629171 Free PMC article.
-
Interpretation of trial-based economic evaluations of musculoskeletal physical therapy interventions.Braz J Phys Ther. 2021 Sep-Oct;25(5):514-529. doi: 10.1016/j.bjpt.2021.06.011. Epub 2021 Jul 21. Braz J Phys Ther. 2021. PMID: 34340933 Free PMC article. Review.
-
Health Economic Publications From the Middle East and North Africa Region: A Scoping Review of the Volume and Methods of Research.Glob J Qual Saf Healthc. 2020 Jun 8;3(2):44-54. doi: 10.36401/JQSH-20-4. eCollection 2020 May. Glob J Qual Saf Healthc. 2020. PMID: 37334147 Free PMC article.
References
-
- OECD. OECD Health Statistics 2014. How Does Brazil Compare? The Organisation for Economic Co-operation and Development (OECD) (2014).
-
- Rajkumar AS, Cavagnero E, Class D, Ferl K. Health Financing Profile—Brazil. Washington: World Bank Group; (2014).
-
- Brasil. Ministério da Saúde. Lei n° 12.401, de 28 de abril de 2011. Altera a Lei n° 8.080, de 19 de setembro de 1990 para dispor sobre a assistência terapêutica e a incorporação de tecnologia em saúde no âmbito do Sistema Único de Saúde. (2011).
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

