Feeling Stress: The Mechanics of Cancer Progression and Aggression
- PMID: 29541636
- PMCID: PMC5835517
- DOI: 10.3389/fcell.2018.00017
Feeling Stress: The Mechanics of Cancer Progression and Aggression
Abstract
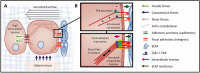
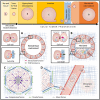
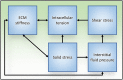
The tumor microenvironment is a dynamic landscape in which the physical and mechanical properties evolve dramatically throughout cancer progression. These changes are driven by enhanced tumor cell contractility and expansion of the growing tumor mass, as well as through alterations to the material properties of the surrounding extracellular matrix (ECM). Consequently, tumor cells are exposed to a number of different mechanical inputs including cell-cell and cell-ECM tension, compression stress, interstitial fluid pressure and shear stress. Oncogenes engage signaling pathways that are activated in response to mechanical stress, thereby reworking the cell's intrinsic response to exogenous mechanical stimuli, enhancing intracellular tension via elevated actomyosin contraction, and influencing ECM stiffness and tissue morphology. In addition to altering their intracellular tension and remodeling the microenvironment, cells actively respond to these mechanical perturbations phenotypically through modification of gene expression. Herein, we present a description of the physical changes that promote tumor progression and aggression, discuss their interrelationship and highlight emerging therapeutic strategies to alleviate the mechanical stresses driving cancer to malignancy.
Keywords: ECM stiffness; cancer progression; cell contractility; mechanical stresses; solid stress; therapeutic targets; tissue tension.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

