Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis
- PMID: 29541790
- PMCID: PMC5861176
- DOI: 10.1007/s00134-018-5126-8
Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis
Abstract
Purpose: Acute kidney injury (AKI) frequently occurs in critically ill patients and often precipitates use of renal replacement therapy (RRT). However, the ideal circumstances for whether and when to start RRT remain unclear. We performed evidence synthesis of the available literature to evaluate the value of biomarkers to predict receipt of RRT for AKI.
Methods: We conducted a PRISMA-guided systematic review and meta-analysis including all trials evaluating biomarker performance for prediction of RRT in AKI. A systematic search was applied in MEDLINE, Embase, and CENTRAL databases from inception to September 2017. All studies reporting an area under the curve (AUC) for a biomarker to predict initiation of RRT were included.
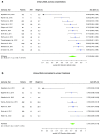
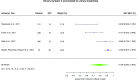
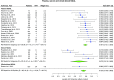
Results: Sixty-three studies comprising 15,928 critically ill patients (median per study 122.5 [31-1439]) met eligibility. Forty-one studies evaluating 13 different biomarkers were included. Of these biomarkers, neutrophil gelatinase-associated lipocalin (NGAL) had the largest body of evidence. The pooled AUCs for urine and blood NGAL were 0.720 (95% CI 0.638-0.803) and 0.755 (0.706-0.803), respectively. Blood creatinine and cystatin C had pooled AUCs of 0.764 (0.732-0.796) and 0.768 (0.729-0.807), respectively. For urine biomarkers, interleukin-18, cystatin C, and the product of tissue inhibitor of metalloproteinase-2 and insulin growth factor binding protein-7 showed pooled AUCs of 0.668 (0.606-0.729), 0.722 (0.575-0.868), and 0.857 (0.789-0.925), respectively.
Conclusion: Though several biomarkers showed promise and reasonable prediction of RRT use for critically ill patients with AKI, the strength of evidence currently precludes their routine use to guide decision-making on when to initiate RRT.
Keywords: Acute kidney injury; Biomarkers; Meta-analysis; Prediction; Renal replacement therapy.
Conflict of interest statement
SJK, AKB, GFL, and HU report no conflict of interest. SMB declares receiving honoraria as a speaker and consultant and research support from Baxter Healthcare Corp. He was a co-investigator in renal biomarker studies. CJW declares receiving honoraria as a speaker for Kedrion, CSL Behring, Baxter, and Grifols and as a consultant for CSL Behring and Grifols. MJ has received honoraria or research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius, and Astute Medical. He was a co-investigator in renal biomarker studies.
Figures



Comment in
-
AKI biomarkers are poor discriminants for subsequent need for renal replacement therapy, but do not disqualify them yet.Intensive Care Med. 2018 Jul;44(7):1156-1158. doi: 10.1007/s00134-018-5151-7. Epub 2018 Apr 12. Intensive Care Med. 2018. PMID: 29651499 No abstract available.
References
-
- Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen A-M, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. - DOI - PubMed
-
- Perinel S, Vincent F, Lautrette A, Dellamonica J, Mariat C, Zeni F, Cohen Y, Tardy B, Souweine B, Darmon M. Transient and persistent acute kidney injury and the risk of hospital mortality in critically ill patients: results of a multicenter cohort study. Crit Care Med. 2015;43:e269–e275. doi: 10.1097/CCM.0000000000001077. - DOI - PubMed
-
- Joannidis M, Druml W, Forni LG, Groeneveld ABJ, Honore PM, Hoste E, Ostermann M, Oudemans-van Straaten HM, Schetz M. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017;43:730–749. doi: 10.1007/s00134-017-4832-y. - DOI - PMC - PubMed
-
- Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel J-M, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard J-D, Dreyfuss D. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. - DOI - PubMed
-
- Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, Boanta A, Gerss J, Meersch M. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

