Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models
- PMID: 29541860
- PMCID: PMC6060757
- DOI: 10.1007/s00424-018-2123-2
Mitochondrial calcium uptake in organ physiology: from molecular mechanism to animal models
Abstract
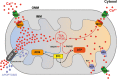
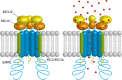
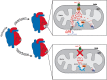
Mitochondrial Ca2+ is involved in heterogeneous functions, ranging from the control of metabolism and ATP production to the regulation of cell death. In addition, mitochondrial Ca2+ uptake contributes to cytosolic [Ca2+] shaping thus impinging on specific Ca2+-dependent events. Mitochondrial Ca2+ concentration is controlled by influx and efflux pathways: the former controlled by the activity of the mitochondrial Ca2+ uniporter (MCU), the latter by the Na+/Ca2+ exchanger (NCLX) and the H+/Ca2+ (mHCX) exchanger. The molecular identities of MCU and of NCLX have been recently unraveled, thus allowing genetic studies on their physiopathological relevance. After a general framework on the significance of mitochondrial Ca2+ uptake, this review discusses the structure of the MCU complex and the regulation of its activity, the importance of mitochondrial Ca2+ signaling in different physiological settings, and the consequences of MCU modulation on organ physiology.
Keywords: Animal models; Heart; Mitochondria Ca2+ uptake; Neurons; Pancreatic β cells; Skeletal muscle.
Figures


References
-
- Alam MR, Groschner LN, Parichatikanond W, Kuo L, Bondarenko AI, Rost R, Waldeck-Weiermair M, Malli R, Graier WF. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J Biol Chem. 2012;287:34445–34454. - PMC - PubMed
-
- Bassi MT, Manzoni M, Bresciani R, Pizzo MT, Della Monica A, Barlati S, Monti E, Borsani G. Cellular expression and alternative splicing of SLC25A23, a member of the mitochondrial Ca2+-dependent solute carrier gene family. Gene. 2005;345:173–182. - PubMed
-
- Bernardi P, Vassanelli S, Veronese P, Colonna R, Szabó I, Zoratti M. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J Biol Chem. 1992;267:2934–2939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

