A Minimal Physiologically Based Pharmacokinetic Model with a Nested Endosome Compartment for Novel Engineered Antibodies
- PMID: 29541870
- PMCID: PMC6486833
- DOI: 10.1208/s12248-017-0183-4
A Minimal Physiologically Based Pharmacokinetic Model with a Nested Endosome Compartment for Novel Engineered Antibodies
Abstract
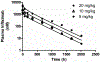
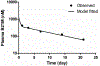
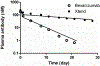
We proposed here a minimal physiologically based pharmacokinetic (mPBPK) model for a group of novel engineered antibodies in mice and humans. These antibodies are designed with altered binding properties of their Fc domain with neonatal Fc receptor (FcRn) or the Fab domain with their cognate targets (recycling antibodies) in acidic endosomes. To enable simulations of such binding features in the change of antibody pharmacokinetics and its target suppression, we nested an endothelial endosome compartment in parallel with plasma compartment based on our previously established mPBPK model. The fluid-phase pinocytosis rate from plasma to endothelial endosomes was reflected by the clearance of antibodies in FcRn dysfunctional humans or FcRn-knockout mice. The endosomal recycling rate of FcRn-bound antibodies was calculated based on the reported endosomal transit time. The nonspecific catabolism in endosomes was fitted using pharmacokinetic data of a human wild-type IgG1 adalimumab in humans and B21M in human FcRn (hFcRn) transgenic mice. The developed model adequately predicted the pharmacokinetics of infliximab, motavizumab, and an Fc variant of motavizumab in humans and the pharmacokinetics of bevacizumab, an Fc variant of bevacizumab, and a recycling antibody PH-IgG1 and its non-pH dependent counterpart NPH-IgG1 in hFcRn transgenic mice. Our proposed model provides a platform for evaluation of the pharmacokinetics and disposition behaviors of Fc-engineered antibodies and recycling antibodies.
Keywords: FcRn; endosome; mPBPK model; pharmacokinetics; recycling antibody.
Conflict of interest statement
Conflict of interest
F. Rode is an employee of Novo Nordisk, Denmark.
Figures
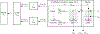
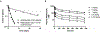

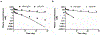
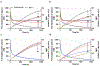
Similar articles
-
A minimal physiologically based pharmacokinetic model to investigate FcRn-mediated monoclonal antibody salvage: Effects of Kon, Koff, endosome trafficking, and animal species.MAbs. 2018 Nov-Dec;10(8):1322-1331. doi: 10.1080/19420862.2018.1506648. Epub 2018 Sep 11. MAbs. 2018. PMID: 30130450 Free PMC article.
-
Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human.J Pharmacokinet Pharmacodyn. 2012 Feb;39(1):67-86. doi: 10.1007/s10928-011-9232-2. Epub 2011 Dec 6. J Pharmacokinet Pharmacodyn. 2012. PMID: 22143261
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
Minimal physiologically-based pharmacokinetic model to investigate the effect of pH dependent FcRn affinity and the endothelial endocytosis on the pharmacokinetics of anti-VEGF humanized IgG1 antibody in cynomolgus monkey.Eur J Pharm Sci. 2018 Dec 1;125:130-141. doi: 10.1016/j.ejps.2018.09.015. Epub 2018 Sep 21. Eur J Pharm Sci. 2018. PMID: 30248389
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
Cited by
-
Modeling Pharmacokinetics and Pharmacodynamics of Therapeutic Antibodies: Progress, Challenges, and Future Directions.Pharmaceutics. 2021 Mar 21;13(3):422. doi: 10.3390/pharmaceutics13030422. Pharmaceutics. 2021. PMID: 33800976 Free PMC article. Review.
-
Development of an mPBPK machine learning framework for early target pharmacology assessment of biotherapeutics.Sci Rep. 2025 Feb 4;15(1):4198. doi: 10.1038/s41598-025-87316-w. Sci Rep. 2025. PMID: 39905178 Free PMC article.
-
A minimal physiologically based pharmacokinetic model to investigate FcRn-mediated monoclonal antibody salvage: Effects of Kon, Koff, endosome trafficking, and animal species.MAbs. 2018 Nov-Dec;10(8):1322-1331. doi: 10.1080/19420862.2018.1506648. Epub 2018 Sep 11. MAbs. 2018. PMID: 30130450 Free PMC article.
-
Prediction of Monoclonal Antibody Pharmacokinetics in Pediatric Populations Using PBPK Modeling and Simulation.Pharmaceutics. 2025 Jul 5;17(7):884. doi: 10.3390/pharmaceutics17070884. Pharmaceutics. 2025. PMID: 40733093 Free PMC article.
-
A minimal physiologically based pharmacokinetic model to study the combined effect of antibody size, charge, and binding affinity to FcRn/antigen on antibody pharmacokinetics.J Pharmacokinet Pharmacodyn. 2024 Oct;51(5):477-492. doi: 10.1007/s10928-023-09899-z. Epub 2024 Feb 24. J Pharmacokinet Pharmacodyn. 2024. PMID: 38400996 Free PMC article.
References
-
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170(6):3528–33. - PubMed
-
- Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, et al. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A. 2006;103(12):5084–9. doi: 10.1073/pnas.0600548103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

