Small heat shock protein speciation: novel non-canonical 44 kDa HspB5-related protein species in rat and human tissues
- PMID: 29542021
- PMCID: PMC6111085
- DOI: 10.1007/s12192-018-0890-5
Small heat shock protein speciation: novel non-canonical 44 kDa HspB5-related protein species in rat and human tissues
Abstract
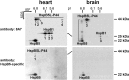
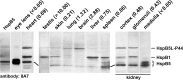
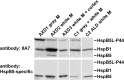
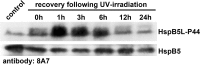
When analyzing small stress proteins of rat and human tissues by electrophoretic methods followed by western blotting, and using the anti-HspB1/anti-HspB5 antibody clone 8A7, we unexpectedly found a protein with a molecular mass of ~44 kDa. On two-dimensional gels, this protein resolved into four distinct species. Electrophoretic and immunological evidence suggests that this 44 kDa protein is a derivative of HspB5, most likely a covalently linked HspB5 dimer. This HspB5-like 44 kDa protein (HspB5L-P44) is particularly abundant in rat heart, brain, and renal cortex and glomeruli. HspB5L-P44 was also found in human brains, including those from patients with Alexander disease, a condition distinguished by cerebral accumulation of HspB5. Gray matter of such a patient contained an elevated amount of HspB5L-P44. A spatial model of structurally ordered dimeric HspB5 α-crystallin domains reveals the exposed and adjacent position of the two peptide segments homologous to the HspB1-derived 8A7 antigen determinant peptide (epitope). This explains the observed extraordinary high avidity of the 8A7 antibody towards HspB5L-P44, as opposed to commonly used HspB5-specific antibodies which recognize other epitopes. This scenario also explains the remarkable fact that no previous study reported the existence of HspB5L-P44 species. Exposure of rat endothelial cells to UV light, an oxidative stress condition, temporarily increased HspB5L-P44, suggesting physiological regulation of the dimerization. The existence of HspB5L-P44 supports the protein speciation discourse and fits to the concept of the protein code, according to which the expression of a given gene is reflected only by the complete set of the derived protein species.
Keywords: Covalently bonded HspB5 dimers; HspB1-/HspB5-specific antibody clone 8A7; Mammals; Protein modification; Protein speciation.
Conflict of interest statement
Rats were housed in animal facilities accredited by the American Association of Laboratory Animal Care, with free access to pelleted food and water. Housing and all procedures were conducted in accordance with the guidelines of the National Institute of Health and were approved by the University of Michigan Committee on Use and Care of Animals (approval nos. 7835 and 7989). Rats were euthanized by inhalation of carbon dioxide in accordance with the American Veterinary Medical Association guidelines on euthanasia.
Figures

References
-
- Behlke J, Dube P, van Heel M, Wieske M, Hayess K, Benndorf R, Lutsch G. Supramolecular structure of the small heat shock protein Hsp25. Progr Colloid Polym Sci. 1995;99:87–93. doi: 10.1007/BFb0114075. - DOI
-
- Benndorf R. HSPB1 and HSPB8 mutations in neuropathies. In: Simon S, Arrigo A-P, editors. Small stress proteins and human diseases. New York: Nova Science Publishers; 2010. pp. 301–324.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

