Pilot randomized controlled trial of a comprehensive smoking cessation intervention for patients with upper aerodigestive cancer undergoing radiotherapy
- PMID: 29542262
- PMCID: PMC6037556
- DOI: 10.1002/hed.25148
Pilot randomized controlled trial of a comprehensive smoking cessation intervention for patients with upper aerodigestive cancer undergoing radiotherapy
Abstract
Background: Smoking among patients with cancer is associated with poor outcomes, however, smoking cessation interventions have had limited success.
Methods: This randomized controlled trial compared a novel smoking cessation intervention ("intervention") with enhanced usual care ("control"). Participants were smokers with head and neck or thoracic malignancies undergoing radiation. Controls received brief counseling. Intervention participants received intensive counseling, pharmacotherapy, text-messaging, and financial incentives. Biochemically confirmed 7-day abstinence at 8 weeks was compared using Fisher's exact t test. Smoking abstinence and intensity were also analyzed using time-series panel regression.
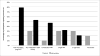
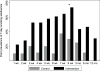
Results: The study population comprised 19 intervention and 10 control participants. More intervention (74%) than control (30%) participants abstained from smoking at 8 weeks (P = .05). Intervention participants were significantly more likely to abstain (adjusted odds ratio [OR] 14.70; 95% confidence interval [CI] 3.56-60.76) and smoked fewer cigarettes (adjusted incidence rate ratio [IRR], 0.16; 95% CI 0.06-0.40) during weeks 1 to 8.
Conclusion: This intervention decreased smoking among patients with upper aerodigestive cancers during radiotherapy.
Keywords: head and neck cancer; lung cancer; radiotherapy; smoking cessation intervention; tobacco dependence.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
None declared.
References
-
- Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Seminars in oncology. 2004;31:726–33. - PubMed
-
- Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123:21S–49S. - PubMed
-
- Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee YC. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head & neck. 2013;35:914–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical