A Reactive Manganese(IV)-Hydroxide Complex: A Missing Intermediate in Hydrogen Atom Transfer by High-Valent Metal-Oxo Porphyrinoid Compounds
- PMID: 29542921
- PMCID: PMC6066600
- DOI: 10.1021/jacs.8b00350
A Reactive Manganese(IV)-Hydroxide Complex: A Missing Intermediate in Hydrogen Atom Transfer by High-Valent Metal-Oxo Porphyrinoid Compounds
Abstract
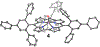
High-valent metal-hydroxide species are invoked as critical intermediates in both catalytic, metal-mediated O2 activation (e.g., by Fe porphyrin in Cytochrome P450) and O2 production (e.g., by the Mn cluster in Photosystem II). However, well-characterized mononuclear MIV(OH) complexes remain a rarity. Herein we describe the synthesis of MnIV(OH)(ttppc) (3) (ttppc = tris(2,4,6-triphenylphenyl) corrole), which has been characterized by X-ray diffraction (XRD). The large steric encumbrance of the ttppc ligand allowed for isolation of 3. The complexes MnV(O)(ttppc) (4) and MnIII(H2O)(ttppc) (1·H2O) were also synthesized and structurally characterized, providing a series of Mn complexes related only by the transfer of hydrogen atoms. Both 3 and 4 abstract an H atom from the O-H bond of 2,4-di- tert-butylphenol (2,4-DTBP) to give a radical coupling product in good yield (3 = 90(2)%, 4 = 91(5)%). Complex 3 reacts with 2,4-DTBP with a rate constant of k2 = 2.73(12) × 104 M-1 s-1, which is ∼3 orders of magnitude larger than 4 ( k2 = 17.4(1) M-1 s-1). Reaction of 3 with a series of para-substituted 2,6-di- tert-butylphenol derivatives (4-X-2,6-DTBP; X = OMe, Me, tBu, H) gives rate constants in the range k2 = 510(10)-36(1.4) M-1 s-1 and led to Hammett and Marcus plot correlations. Together with kinetic isotope effect measurements, it is concluded that O-H cleavage occurs by a concerted H atom transfer (HAT) mechanism and that the MnIV(OH) complex is a much more powerful H atom abstractor than the higher-valent MnV(O) complex, or even some FeIV(O) complexes.
Figures


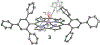

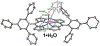





Similar articles
-
Factors Affecting Hydrogen Atom Transfer Reactivity of Metal-Oxo Porphyrinoid Complexes.Acc Chem Res. 2018 Nov 20;51(11):2641-2652. doi: 10.1021/acs.accounts.8b00414. Epub 2018 Nov 7. Acc Chem Res. 2018. PMID: 30403479 Free PMC article.
-
Hydrogen Atom Abstraction by High-Valent Fe(OH) versus Mn(OH) Porphyrinoid Complexes: Mechanistic Insights from Experimental and Computational Studies.Inorg Chem. 2019 Dec 16;58(24):16761-16770. doi: 10.1021/acs.inorgchem.9b02923. Epub 2019 Dec 5. Inorg Chem. 2019. PMID: 31804814 Free PMC article.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
-
Synthesis and structural characterization of a series of Mn(III)OR complexes, including a water-soluble Mn(III)OH that promotes aerobic hydrogen-atom transfer.Inorg Chem. 2013 Nov 4;52(21):12383-93. doi: 10.1021/ic401234t. Epub 2013 Oct 24. Inorg Chem. 2013. PMID: 24156315 Free PMC article.
-
High-valent iron and manganese complexes of corrole and porphyrin in atom transfer and dioxygen evolving catalysis.Dalton Trans. 2011 Apr 14;40(14):3435-44. doi: 10.1039/c0dt01341b. Epub 2011 Jan 31. Dalton Trans. 2011. PMID: 21279237 Review.
Cited by
-
Involvement of high-valent manganese-oxo intermediates in oxidation reactions: realisation in nature, nano and molecular systems.Nano Converg. 2018;5(1):18. doi: 10.1186/s40580-018-0150-5. Epub 2018 Jul 4. Nano Converg. 2018. PMID: 30101051 Free PMC article. Review.
-
Factors Affecting Hydrogen Atom Transfer Reactivity of Metal-Oxo Porphyrinoid Complexes.Acc Chem Res. 2018 Nov 20;51(11):2641-2652. doi: 10.1021/acs.accounts.8b00414. Epub 2018 Nov 7. Acc Chem Res. 2018. PMID: 30403479 Free PMC article.
-
Nuclear Resonance Vibrational Spectroscopic Definition of the Fe(IV)2 Intermediate Q in Methane Monooxygenase and Its Reactivity.J Am Chem Soc. 2021 Oct 6;143(39):16007-16029. doi: 10.1021/jacs.1c05436. Epub 2021 Sep 27. J Am Chem Soc. 2021. PMID: 34570980 Free PMC article.
-
Mechanistic Dichotomy in Proton-Coupled Electron-Transfer Reactions of Phenols with a Copper Superoxide Complex.J Am Chem Soc. 2019 Apr 3;141(13):5470-5480. doi: 10.1021/jacs.9b00466. Epub 2019 Mar 25. J Am Chem Soc. 2019. PMID: 30907590 Free PMC article.
-
Formation and Reactivity of an Elusive Monomeric Mn(IV)-Oxo Species Inside a Cavitand Pore.J Am Chem Soc. 2025 Aug 27;147(34):30647-30660. doi: 10.1021/jacs.5c02637. Epub 2025 Aug 7. J Am Chem Soc. 2025. PMID: 40771048 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

