Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users
- PMID: 29543325
- PMCID: PMC6513394
- DOI: 10.1002/14651858.CD011481.pub2
Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users
Abstract
Background: Prolonged treatment with benzodiazepines is common practice despite clinical recommendations of short-term use. Benzodiazepines are used by approximately 4% of the general population, with increased prevalence in psychiatric populations and the elderly. After long-term use it is often difficult to discontinue benzodiazepines due to psychological and physiological dependence. This review investigated if pharmacological interventions can facilitate benzodiazepine tapering.
Objectives: To assess the benefits and harms of pharmacological interventions to facilitate discontinuation of chronic benzodiazepine use.
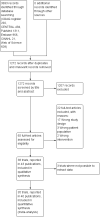
Search methods: We searched the following electronic databases up to October 2017: Cochrane Drugs and Alcohol Group's Specialised Register of Trials, CENTRAL, PubMed, Embase, CINAHL, and ISI Web of Science. We also searched ClinicalTrials.gov, the WHO ICTRP, and ISRCTN registry, and checked the reference lists of included studies for further references to relevant randomised controlled trials.
Selection criteria: We included randomised controlled trials comparing pharmacological treatment versus placebo or no intervention or versus another pharmacological intervention in adults who had been treated with benzodiazepines for at least two months and/or fulfilled criteria for benzodiazepine dependence (any criteria).
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
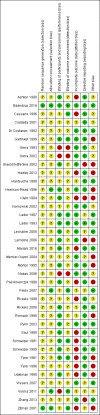
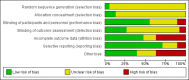
Main results: We included 38 trials (involving 2543 participants), but we could only extract data from 35 trials with 2295 participants. Many different interventions were studied, and no single intervention was assessed in more than four trials. We extracted data on 18 different comparisons. The risk of bias was high in all trials but one. Trial Sequential Analysis showed imprecision for all comparisons.For benzodiazepine discontinuation, we found a potential benefit of valproate at end of intervention (1 study, 27 participants; risk ratio (RR) 2.55, 95% confidence interval (CI) 1.08 to 6.03; very low-quality evidence) and of tricyclic antidepressants at longest follow-up (1 study, 47 participants; RR 2.20, 95% CI 1.27 to 3.82; low-quality evidence).We found potentially positive effects on benzodiazepine withdrawal symptoms of pregabalin (1 study, 106 participants; mean difference (MD) -3.10 points, 95% CI -3.51 to -2.69; very low-quality evidence), captodiame (1 study, 81 participants; MD -1.00 points, 95% CI -1.13 to -0.87; very low-quality evidence), paroxetine (2 studies, 99 participants; MD -3.57 points, 95% CI -5.34 to -1.80; very low-quality evidence), tricyclic antidepressants (1 study, 38 participants; MD -19.78 points, 95% CI -20.25 to -19.31; very low-quality evidence), and flumazenil (3 studies, 58 participants; standardised mean difference -0.95, 95% CI -1.71 to -0.19; very low-quality evidence) at end of intervention. However, the positive effect of paroxetine on benzodiazepine withdrawal symptoms did not persist until longest follow-up (1 study, 54 participants; MD -0.13 points, 95% CI -4.03 to 3.77; very low-quality evidence).The following pharmacological interventions reduced symptoms of anxiety at end of intervention: carbamazepine (1 study, 36 participants; MD -6.00 points, 95% CI -9.58 to -2.42; very low-quality evidence), pregabalin (1 study, 106 participants; MD -4.80 points, 95% CI -5.28 to -4.32; very low-quality evidence), captodiame (1 study, 81 participants; MD -5.70 points, 95% CI -6.05 to -5.35; very low-quality evidence), paroxetine (2 studies, 99 participants; MD -6.75 points, 95% CI -9.64 to -3.86; very low-quality evidence), and flumazenil (1 study, 18 participants; MD -1.30 points, 95% CI -2.28 to -0.32; very low-quality evidence).Two pharmacological treatments seemed to reduce the proportion of participants that relapsed to benzodiazepine use: valproate (1 study, 27 participants; RR 0.31, 95% CI 0.11 to 0.90; very low-quality evidence) and cyamemazine (1 study, 124 participants; RR 0.33, 95% CI 0.14 to 0.78; very low-quality evidence). Alpidem decreased the proportion of participants with benzodiazepine discontinuation (1 study, 25 participants; RR 0.41, 95% CI 0.17 to 0.99; number needed to treat for an additional harmful outcome (NNTH) 2.3 participants; low-quality evidence) and increased the occurrence of withdrawal syndrome (1 study, 145 participants; RR 4.86, 95% CI 1.12 to 21.14; NNTH 5.9 participants; low-quality evidence). Likewise, magnesium aspartate decreased the proportion of participants discontinuing benzodiazepines (1 study, 144 participants; RR 0.80, 95% CI 0.66 to 0.96; NNTH 5.8; very low-quality evidence).Generally, adverse events were insufficiently reported. Specifically, one of the flumazenil trials was discontinued due to severe panic reactions.
Authors' conclusions: Given the low or very low quality of the evidence for the reported outcomes, and the small number of trials identified with a limited number of participants for each comparison, it is not possible to draw firm conclusions regarding pharmacological interventions to facilitate benzodiazepine discontinuation in chronic benzodiazepine users. Due to poor reporting, adverse events could not be reliably assessed across trials. More randomised controlled trials are required with less risk of systematic errors ('bias') and of random errors ('play of chance') and better and full reporting of patient-centred and long-term clinical outcomes. Such trials ought to be conducted independently of industry involvement.
Conflict of interest statement
LB is the sponsor‐investigator of one of the studies included in this review (Baandrup 2016). A review author independent of this trial acted as the second review author, thus ensuring unbiased data extraction and 'Risk of bias' assessment.
BE has received lecture fees from Bristol‐Myers Squibb, Otsuka Pharma Scandinavia AB, and Eli Lilly and Company and is part of the Advisory Board of Eli Lilly Danmark A/S, Janssen‐Cilag A/S, and Takeda Pharmaceutical Company Ltd.
BG is leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research, CINS. CINS is independent of H. Lundbeck A/S. The grant was awarded based on international scientific review. BG is part of the study group behind the clinical trial stated by LB in her declaration of interest.
JL, CG, and JR have no known conflicts of interest.
Figures










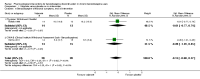











































































Update of
- doi: 10.1002/14651858.CD011481
References
References to studies included in this review
Ashton 1990 {published data only}
-
- Ashton CH, Rawlins MD, Tyrer SP. A double‐blind placebo‐controlled study of buspirone in diazepam withdrawal in chronic benzodiazepine users. British Journal of Psychiatry 1990;157:232‐8. - PubMed
Baandrup 2016 {published data only}
-
- Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well‐being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add‐on melatonin versus placebo. European Archives of Psychiatry and Clinical Neuroscience 2017;267(2):163‐71. [PUBMED: 27400927] - PubMed
-
- Baandrup L, Glenthoj BY, Jennum PJ. Objective and subjective sleep quality: melatonin versus placebo add‐on treatment in patients with schizophrenia or bipolar disorder withdrawing from long‐term benzodiazepine use. Psychiatry Research 2016;240:163‐9. [PUBMED: 27107670] - PubMed
-
- Baandrup L, Lindschou J, Winkel P, Gluud C, Glenthoj BY. Prolonged‐release melatonin versus placebo for benzodiazepine discontinuation in patients with schizophrenia or bipolar disorder: a randomised, placebo‐controlled, blinded trial. World Journal of Biological Psychiatry 2016;17(7):514‐24. [PUBMED: 26086792] - PubMed
Cassano 1996 {published data only}
-
- Cassano GB, Petracca A, Borghi C, Chiroli S, Didoni G, Garreau M. A randomized, double‐blind study of alpidem vs placebo in the prevention and treatment of benzodiazepine withdrawal syndrome. European Psychiatry 1996;11(2):93‐9. - PubMed
Cialdella 2001 {published data only}
-
- Cialdella P, Boissel JP, Belon P, the ASTRHO group. Homeopathic specialties as a substitute for benzodiazepines: a double‐blind vs. placebo study. Thérapie 2001;56:397‐402. - PubMed
Di Costanzo 1992 {published data only}
-
- Costanzo E, Rovea A. The prophylaxis of benzodiazepine withdrawal syndrome in the elderly: the effectiveness of carbamazepine. A double‐blind study vs. placebo. Minerva Psichiatrica 1992;33:301‐4. - PubMed
Garfinkel 1999 {published data only}
-
- Garfinkel D, Zisapel N, Wainstein J, Laudon M. Facilitation of benzodiazepine discontinuation by melatonin: a new clinical approach. Archives of Internal Medicine 1999;159(20):2456‐60. - PubMed
Gerra 1993 {published data only}
-
- Gerra G, Marcato A, Caccavari R, Fertonani‐Affini G, Fontanesi B, Zaimovic A, et al. Effectiveness of flumazenil (Ro 15‐1788) in the treatment of benzodiazepine withdrawal. Current Therapeutic Research ‐ Clinical and Experimental 1993;54(5):580‐7.
Gerra 2002 {published data only}
-
- Gerra G, Zaimovic A, Giusti F, Moi G, Brewer C. Intravenous flumazenil versus oxazepam tapering in the treatment of benzodiazepine withdrawal: a randomized, placebo‐controlled study. Addiction Biology 2002;7(4):385‐95. - PubMed
GlaxoSmithKline 2002 {unpublished data only}
-
- GlaxoSmithKline. Clinical comparison of paroxetine and placebo on the symptoms emerging during the taper phase of a chronic benzodiazepine treatment, in patients suffering from a variety of anxiety disorders. GSK ‐ Clinical Study Register (www.gsk‐clinicalstudyregister.com) 2002.
Hadley 2012 {published data only}
-
- Szczypa P, Hadley SJ, Donevan S, Mandel FS, Leon T. P.4.a.016 Switching from long‐term benzodiazepine therapy to pregabalin in patients with generalised anxiety disorder (GAD). European Neuropsychopharmacology 2009;19:S594‐5. [DOI: 10.1016/S0924-977X(09)70952-6] - DOI
Hantouche 1998 {published data only}
-
- Hantouche EG, Guelfi JD, Comet D. Discontinuation of long‐term benzodiazepine use: double‐blind controlled study of α‐β L‐aspartate magnesium versus placebo in 144 chronic users of benzodiazepines [α‐β L‐aspartate de magnésium dans l'arrêt de la consommation chronique des benzodiazépines: étude contrôlée en double aveugle versus placebo]. L'encéphale 1998;XXIV:469‐79. - PubMed
-
- Hantouche EG, Jacob L, Comet D, Guelfi JD. Discontinuation of long‐term benzodiazepine use: predictive model of success in a double‐blind, controlled‐study. 150th Annual Meeting of the American Psychiatric Association, 1997 May 17‐22; San Diego, (CA). 1997.
Harrison‐Read 1996 {published data only}
-
- Harrison‐Read PE, Tyrer P, Lawson C, Lack S, Fernandes C, File SE. Flumazenil‐precipitated panic and dysphoria in patients dependent on benzodiazepines: a possible aid to abstinence. Journal of Psychopharmacology 1996;10(2):89‐97. - PubMed
Klein 1994 {published data only}
-
- Klein E, Colin V, Stolk J, Lenox RH. Alprazolam withdrawal in patients with panic disorder and generalized anxiety disorder: vulnerability and effect of carbamazepine. American Journal of Psychiatry 1994;151(12):1760‐6. - PubMed
Kornowski 2002 {published data only}
-
- Kornowski J. The comparison between tianeptine and carbamazepine in benzodiazepines withdrawal syndrome. Psychiatria Polska 2002;6(Suppl):311‐8. - PubMed
Lader 1987 {published data only}
-
- Lader M, Olajide D. A comparison of buspirone and placebo in relieving benzodiazepine withdrawal symptoms. Journal of Clinical Psychopharmacology 1987;7(1):11‐5. - PubMed
Lader 1993 {published data only}
-
- Lader M, Farr I, Morton S. A comparison of alpidem and placebo in relieving benzodiazepine withdrawal symptoms. International Clinical Psychopharmacology 1993;8(1):31‐6. - PubMed
Lecrubier 2005 {published data only}
-
- Lecrubier Y, Fessard N. Benzodiazepine discontinuation in chronic users: a double‐blind trial of lithium gluconate vs placebo [Arrêt des benzodiazépines chez des consommateurs chronique: un essai en double insu du gluconate de lithium vs placebo]. Annales Médico Phychologiques 2005;163:24‐9.
Lemoine 2006 {published data only}
-
- Lemoine P, Kermadi I, Garcia‐Acosta S, Garay RP, Dib M. Double‐blind, comparative study of cyamemazine vs. bromazepam in the benzodiazepine withdrawal syndrome. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2006;30(1):131‐7. - PubMed
Mariani 2016 {published data only}
Mercier‐Guyon 2004 {published data only}
Morton 1995 {published data only}
-
- Morton S, Lader M. Buspirone treatment as an aid to benzodiazepine withdrawal. Journal of Psychopharmacology 1995;9(4):331‐5. - PubMed
Nakao 2006 {published data only}
-
- Nakao M, Takeuchi T, Nomura K, Teramoto T, Yano E. Clinical application of paroxetine for chronic benzodiazepine users at an internal medicine clinic. Therapeutic Research 2006;27(5):859‐67. - PubMed
-
- Nakao M, Takeuchi T, Nomura K, Teramoto T, Yano E. Clinical application of paroxetine for tapering benzodiazepine use in non‐major‐depressive outpatients visiting an internal medicine clinic. Psychiatry and Clinical Neurosciences 2006;60(5):605‐10. - PubMed
Pat‐Horenczyk 1998 {published data only}
Peles 2007 {published data only}
-
- Peles E, Hetzroni T, Bar‐Hamburger R, Adelson M, Schreiber S. Melatonin for perceived sleep disturbances associated with benzodiazepine withdrawal among patients in methadone maintenance treatment: a double‐blind randomized clinical trial. Addiction 2007;102(12):1947‐53. - PubMed
Rickels 1999 {published data only}
-
- Rickels K, Schweizer E, Garcia Espana F, Case, G, DeMartinis N, Greenblatt D. Trazodone and valproate in patients discontinuing long‐term benzodiazepine therapy: effects on withdrawal symptoms and taper outcome. Psychopharmacology 1999;141(1):1‐5. - PubMed
Rickels 2000 {published data only}
-
- Rickels K, DeMartinis N, Garcia‐Espana F, Greenblatt DJ, Mandos LA, Rynn M. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long‐term benzodiazepine therapy. American Journal of Psychiatry 2000;157(12):1973‐9. - PubMed
Romach 1998 {published data only}
-
- Romach MK, Kaplan HL, Busto UE, Somer G, Sellers EM. A controlled trial of ondansetron, a 5‐HT3 antagonist, in benzodiazepine discontinuation. Journal of Clinical Psychopharmacology 1998;18(2):121‐31. - PubMed
Rynn 2003 {published data only}
-
- Rynn M, Garcia‐Espana F, Greenblatt DJ, Mandos LA, Schweizer E, Rickels K. Imipramine and buspirone in patients with panic disorder who are discontinuing long‐term benzodiazepine therapy. Journal of Clinical Psychopharmacology 2003;23(5):505‐8. - PubMed
Saul 1989 {published data only}
-
- Saul PA, Korlipara K, Presley P. A randomised, multicentre, double‐blind, comparison of atenolol and placebo in the control of benzodiazepine withdrawal symptoms. Acta Therapeutica 1989;15(2):117‐23.
Schweizer 1991 {published data only}
-
- Schweizer E, Rickels, K, Case WG, Greenblatt DJ. Carbamazepine treatment in patients discontinuing long‐term benzodiazepine therapy. Effects on withdrawal severity and outcome. Archives of General Psychiatry 1991;48(5):448‐52. - PubMed
Schweizer 1995 {published data only}
-
- Schweizer E, Case WG, Garcia‐Espana F, Greenblatt DJ, Rickels K. Progesterone co‐administration in patients discontinuing long‐term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology 1995;117(4):424‐9. - PubMed
Tyrer 1981 {published data only}
-
- Tyrer P, Rutherford D, Huggett T. Benzodiazepine withdrawal symptoms and propranolol. Lancet 1981;1(8219):520‐2. - PubMed
Tyrer 1996 {published data only}
-
- Tyrer P, Ferguson B, Hallstrom C, Michie M, Tyrer S, Cooper S, et al. A controlled trial of dothiepin and placebo in treating benzodiazepine withdrawal symptoms. British Journal of Psychiatry 1996;168(4):457‐61. - PubMed
Udelman 1990 {published data only}
-
- Udelman HD, Udelman DL. Concurrent use of buspirone in anxious patients during withdrawal from alprazolam therapy. Journal of Clinical Psychiatry 1990;51 Suppl:46‐50. - PubMed
Vissers 2007 {published data only}
Vorma 2011 {published data only}
-
- Vorma H, Katila H. Effect of valproate on benzodiazepine withdrawal severity in opioid‐dependent subjects: a pilot study. Heroin Addiction and Related Clinical Problems 2011;13(1):15‐20.
Zhang 2013 {published data only}
-
- Zhang H, Jiang X, Ma M, Zhang J. A control study on treatment for benzodiazepine dependence with trazodone. Chinese Journal of Contemporary Neurology and Neurosurgery 2013;13(5):411‐5.
Zitman 2001 {published data only}
-
- Zitman FG, Couvee JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper‐off: report on behalf of the Dutch Chronic Benzodiazepine Working Group. British Journal of Psychiatry 2001;178:317‐24. - PubMed
References to studies excluded from this review
Allain 1998 {published data only}
-
- Allain H, Coz F, Borderies P, Schuck S, Giclais B, Patat A, et al. Use of zolpidem 10 mg as a benzodiazepine substitute in 84 patients with insomnia. Human Psychopharmacology 1998;13(8):551‐9.
Avedisova 2007 {published data only}
-
- Avedisova AS, Iastrebov DV. Use of anxiolytic atarax as a substitutive drug for benzodiazepine tranquilizers. Zhurnal Nevrologii i psikhiatrii imeni S.S. Korsakova 2007;107(3):37‐41. - PubMed
Bobes 2012 {published data only}
-
- Bobes J, Rubio G, Teran A, Cervera G, Lopez‐Gomez V, Vilardaga I, et al. Pregabalin for the discontinuation of long‐term benzodiazepines use: an assessment of its effectiveness in daily clinical practice. European Psychiatry 2012;27(4):301‐7. - PubMed
Bourgeois 2014 {published data only}
-
- Bourgeois J, Elseviers MM, Bortel L, Petrovic M, Vander Stichele RH. Feasibility of discontinuing chronic benzodiazepine use in nursing home residents: a pilot study. European Journal of Clinical Pharmacology 2014;17(10):1251‐60. - PubMed
Cantopher 1990 {published data only}
-
- Cantopher T, Olivieri S, Cleave N, Edwards JG. Chronic benzodiazepine dependence. A comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. British Journal of Psychiatry 1990;156:406‐11. - PubMed
Cohen‐Mansfield 1999 {published data only}
-
- Cohen‐Mansfield J, Lipson S, Werner P, Billig N, Taylor L, Woosley R. Withdrawal of haloperidol, thioridazine, and lorazepam in the nursing home: a controlled, double‐blind study. Archives of Internal Medicine 1999;159(15):1733‐40. - PubMed
Declerck 1999 {published data only}
-
- Declerck A, Smits M. Zolpidem, a valuable alternative to benzodiazepine hypnotics for chronic insomnia?. Journal of International Medical Research 1999;27(6):253‐63. - PubMed
Emara 2009 {published data only}
-
- Emara A, Elgharabawy R. Evaluation of escitalopram in the treatment of benzodiazepines withdrawal syndrome. Basic and Clinical Pharmacology and Toxicology 2009;105(Suppl 1):66.
Garcia‐Borreguero 1992 {published data only}
-
- Garcia‐Borreguero D, Bronisch T, Apelt S, Yassouridis J, Emrich HM. Treatment of benzodiazepine withdrawal symptoms with carbamazepine. Pharmacopsychiatry 1992;25:100. - PubMed
Hallstrom 1988 {published data only}
-
- Hallstrom C, Crouch G, Robson M, Shine P. The treatment of tranquilizer dependence by propranolol. Postgraduate Medical Journal 1988;64 Suppl 2:40‐4. - PubMed
Isaka 2009 {published data only}
-
- Isaka M. Withdrawal symptoms of benzodiazepines in panic disorder patients' pharmacotherapy. Journal of the Osaka City Medical Center 2009;58(1‐2):11‐20.
Lahteenmaki 2014 {published data only}
Lemoine 1997 {published data only}
-
- Lemoine P, Touchon J, Billardon M. Comparison of 6 different methods for lorazepam withdrawal. A controlled study, hydroxyzine versus placebo. Encephale 1997;23(4):290‐9. - PubMed
Lopatko 2006 {published data only}
-
- Lopatko O, Morefield K, Danz C, Davies J, Ali R, White JM. Reducing benzodiazepine consumption in opioid maintenance therapy patients: A controlled clinical trial. Proceedings of the 68th Annual Scientific Meeting of the College on Problems of Drug Dependence. 2006:20.
Nakajima 2007 {published data only}
-
- Nakajima S, Uchida H, Suzuki T, Tomita M, Tsunoda K, Kitta M, et al. An open‐label trial of discontinuing benzodiazepines in patients with chronic schizophrenia. Journal of Clinical Psychopharmacology 2007;27(4):401‐3. - PubMed
Petrovic 2002 {published data only}
-
- Petrovic M, Pevernagie D, Mariman A, Maele G, Afschrift M. Fast withdrawal from benzodiazepines in geriatric inpatients: a randomised double‐blind, placebo‐controlled trial. European Journal of Clinical Pharmacology 2002;57:759‐64. - PubMed
Rocco 1992 {published data only}
-
- Rocco PL, Giavedoni A, Pacella G. Withdrawal from benzodiazepines in a hospital setting: an open trial with buspirone. Current Therapeutic Research ‐ Clinical and Experimental 1992;52(3):386‐9.
Rubio 2009 {published data only}
-
- Rubio G, Bobes J, Cervera G, Teran A, Perez M, Lopez‐Gomez V, et al. Benzodiazepines use withdrawal tapering gradually with pregabalin: findings from the medical practice. European Neuropsychopharmacology 2009;19:S652.
Saxon 1997 {published data only}
-
- Saxon L, Hjemdahl P, Hiltunen AJ, Borg S. Effects of flumazenil in the treatment of benzodiazepine withdrawal ‐ a double‐blind pilot study. Psychopharmacology 1997;131(2):153‐60. - PubMed
Shapiro 1995 {published data only}
-
- Shapiro CM, Sherman D, Peck DF. Withdrawal from benzodiazepines by initially switching to zopiclone. European Psychiatry 1995;10:S145‐51. - PubMed
Vescovi 1987 {published data only}
-
- Vescovi PP, Gerra G, Ippolito L. Nicotinic acid effectiveness in the treatment of benzodiazepine withdrawal. Current Therapeutic Research ‐ Clinical and Experimental 1987;41(6):1017‐21.
Weizman 2003 {published data only}
-
- Weizman T, Gelkopf M, Melamed Y, Adelson M, Bleich A. Treatment of benzodiazepine dependence in methadone maintenance treatment patients: a comparison of two therapeutic modalities and the role of psychiatric comorbidity. Australian and New Zealand Journal of Psychiatry 2003;37(4):458‐63. - PubMed
Additional references
Ashton 2005
-
- Ashton H. The diagnosis and management of benzodiazepine dependence. Current Opinion in Psychiatry 2005;18(3):249‐55. [PUBMED: 16639148] - PubMed
Baandrup 2015
Bachhuber 2016
Baldwin 2013
-
- Baldwin DS, Aitchison K, Bateson A, Curran HV, Davies S, Leonard B, et al. Benzodiazepines: risks and benefits. A reconsideration. Journal of Psychopharmacology 2013;27(11):967‐71. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Barker 2004
-
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long‐term benzodiazepine use: a meta‐analysis. CNS Drugs 2004;18(1):37‐48. - PubMed
Billioti 2012
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Buscemi 2007a
Chan 2015
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ (accessed 25 March 2017).
Darker 2015
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dell'osso 2013
-
- Dell'osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. European Psychiatry: the Journal of the Association of European Psychiatrists 2013;28(1):7‐20. [PUBMED: 22521806] - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
Denis 2006
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Egger 1997
Gallacher 2012
-
- Gallacher J, Elwood P, Pickering J, Bayer A, Fish M, Ben‐Shlomo Y. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). Journal of Epidemiology and Community Health 2012;66(10):869‐73. - PubMed
Gisev 2011
-
- Gisev N, Hartikainen S, Chen TF, Korhonen M, Bell JS. Mortality associated with benzodiazepines and benzodiazepine‐related drugs among community‐dwelling older people in Finland: a population‐based retrospective cohort study. Canadian Journal of Psychiatry 2011;56(6):377‐81. - PubMed
Glass 2005
Hausken 2007
-
- Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle‐aged population. Pharmacoepidemiology and Drug Safety 2007;16(8):913‐8. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huthwaite 2013
-
- Huthwaite MA, Andersson V, Stanley J, Romans SE. Hypnosedative prescribing in outpatient psychiatry. International Clinical Psychopharmacology 2013;28(4):157‐63. - PubMed
ICH GCP
-
- International Council for Harmonisation (ICH) Topic E 6 (R1) Guideline for Good Clinical Practice. ichgcp.net/pdf/ich‐gcp‐en.pdf (accessed prior to 10 February 2018).
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] - PubMed
Ioannidis 2009
-
- Ioannidis JP. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Archives of Internal Medicine 2009; Vol. 169, issue 19:1737‐9. [PUBMED: 19858427] - PubMed
Islam 2014
-
- Islam MM, Conigrave KM, Day CA, Nguyen Y, Haber PS. Twenty‐year trends in benzodiazepine dispensing in the Australian population. Internal Medicine Journal 2014;44(1):57‐64. - PubMed
Jakobsen 2014
Jaussent 2013
Jones 2014
Kripke 1998
-
- Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biological Psychiatry 1998;43(9):687‐93. - PubMed
Kripke 2012
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lundh 2017
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Mallon 2009
-
- Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality?. Sleep Medicine 2009;10(3):279‐86. - PubMed
Manchikanti 2008
-
- Manchikanti L, Singh A. Therapeutic opioids: a ten‐year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008;11(2 Suppl):S63‐88. [PUBMED: 18443641] - PubMed
Manchikanti 2012
-
- Manchikanti L, Helm S Jr, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain Physician 2012;15(3 Suppl):ES9‐38. [PUBMED: 22786464] - PubMed
O'Brien 2005
-
- O'Brien CP. Benzodiazepine use, abuse, and dependence. Journal of Clinical Psychiatry 2005;66 Suppl 2:28‐33. - PubMed
Parr 2009
-
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta‐analysis. Addiction 2009;104(1):13‐24. - PubMed
Parrino 1996
-
- Parrino L, Terzano MG. Polysomnographic effects of hypnotic drugs. A review. Psychopharmacology 1996;126(1):1‐16. - PubMed
Paulose‐Ram 2007
-
- Paulose‐Ram R, Safran MA, Jonas BS, Gu Q, Orwig D. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiology and Drug Safety 2007;16(5):560‐70. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schroll 2015
Schulz 2011
-
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery (London, England) 2011;9(8):672‐7. [PUBMED: 22019563] - PubMed
Skolnick 2018
-
- Skolnick P. The opioid epidemic: crisis and solutions. Annual Review of Pharmacology and Toxicology 2018;58:143‐59. [PUBMED: 28968188] - PubMed
Smink 2010
-
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systematic literature review. CNS Drugs 2010;24(8):639‐53. - PubMed
Sonnenberg 2012
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011a
Thorlund 2011b
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf (accessed 19 March 2014).
Tsimtsiou 2009
Turner 2014
Vinkers 2012
Voshaar 2006
-
- Voshaar RC, Couvee JE, Balkom AJ, Mulder PG, Zitman FG. Strategies for discontinuing long‐term benzodiazepine use: meta‐analysis. British Journal of Psychiatry 2006;189:213‐20. - PubMed
Warner 2009
-
- Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999‐2006. NCHS Data Brief 2009, (22):1‐8. [PUBMED: 19796521] - PubMed
Webster 2011
-
- Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, et al. An analysis of the root causes for opioid‐related overdose deaths in the United States. Pain Medicine (Malden, Mass.) 2011;12 Suppl 2:S26‐35. [PUBMED: 21668754] - PubMed
Weich 2014
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wetterslev 2017
Woolcott 2009
-
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons. Archives of Internal Medicine 2009;169(21):1952‐60. - PubMed
Wu 2009
-
- Wu CS, Wang SC, Chang IS, Lin KM. The association between dementia and long‐term use of benzodiazepine in the elderly: nested case‐control study using claims data. American Journal of Geriatric Psychiatry 2009;17(7):614‐20. - PubMed
Zandstra 2004
-
- Zandstra SM, Rijswijk E, Rijnders CA, Lisdonk EH, Bor JH, Weel C, et al. Long‐term benzodiazepine users in family practice: differences from short‐term users in mental health, coping behaviour and psychological characteristics. Family Practice 2004;21(3):266‐9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

