Calcitonin gene-related peptide hyperpolarizes mouse pulmonary artery endothelial tubes through KATP channel activation
- PMID: 29543503
- PMCID: PMC6139654
- DOI: 10.1152/ajplung.00044.2018
Calcitonin gene-related peptide hyperpolarizes mouse pulmonary artery endothelial tubes through KATP channel activation
Abstract
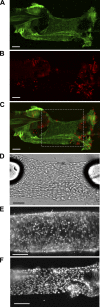
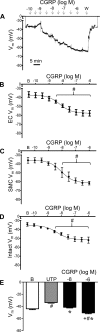
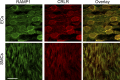
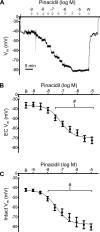
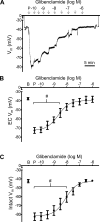
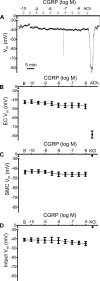
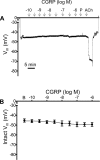
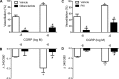
The sensory neurotransmitter calcitonin gene-related peptide (CGRP) is associated with vasodilation of systemic arteries through activation of ATP-sensitive K+ (KATP) channels in smooth muscle cells (SMCs); however, its effects on endothelial cell (EC) membrane potential ( Vm) are unresolved. In pulmonary arteries (PAs) of C57BL/6J mice, we questioned whether CGRP would hyperpolarize ECs as well as SMCs. Intact PAs were isolated and immunostained for CGRP to confirm sensory innervation; vessel segments (1-2 mm long, ∼150 µm diameter) with intact or denuded endothelium were cannulated and pressurized to 16 cmH2O at 37°C. Increasing concentrations (10-10-10-6 M) of CGRP progressively dilated PAs preconstricted with UTP (10-5 M); SMCs hyperpolarized similarly (Δ Vm ∼20 mV) before and after endothelial denudation. To study native intact PA ECs, SMCs were dissociated to isolate endothelial tubes, and their integrity was confirmed by vital dye uptake, nuclear staining, and reproducible electrical and intracellular Ca2+ responses to acetylcholine (10-5 M) over 2 h. Increasing [CGRP] hyperpolarized ECs in a manner similar to SMCs, with each cell layer demonstrating robust immunostaining for CGRP receptor proteins. Increasing concentrations (10-10-10-6 M) of pinacidil, a KATP channel agonist, resulted in progressive hyperpolarization of SMCs of intact PAs (Δ Vm ∼30 mV), which was blocked by glibenclamide (10-6 M), as was hyperpolarization of ECs and SMCs to CGRP. Inhibition of protein kinase A with protein kinase inhibitor (10-5 M) also inhibited hyperpolarization to CGRP. We demonstrate [CGRP]-dependent hyperpolarization of ECs for the first time while validating freshly isolated PA endothelial tubes as an experimental model. Redundant electrical signaling to CGRP in ECs and SMCs implies an integral role for KATP channels in PA dilation.
Keywords: hyperpolarization; lung blood flow; protein kinase A; sensory nerves; vasodilation.
Figures





Similar articles
-
Differential hyperpolarization to substance P and calcitonin gene-related peptide in smooth muscle versus endothelium of mouse mesenteric artery.Microcirculation. 2021 Nov;28(8):e12733. doi: 10.1111/micc.12733. Epub 2021 Oct 21. Microcirculation. 2021. PMID: 34633728 Free PMC article.
-
Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A.J Physiol. 1994 Feb 15;475(1):9-13. doi: 10.1113/jphysiol.1994.sp020045. J Physiol. 1994. PMID: 8189394 Free PMC article.
-
Inhibitory transmitter action of calcitonin gene-related peptide in guinea-pig ureter via activation of glibenclamide-sensitive K channels.Br J Pharmacol. 1994 Oct;113(2):588-92. doi: 10.1111/j.1476-5381.1994.tb17030.x. Br J Pharmacol. 1994. PMID: 7834212 Free PMC article.
-
Role of potassium channels in the vascular response to endogenous and pharmacological vasodilators.Blood Vessels. 1991;28(1-3):147-53. doi: 10.1159/000158854. Blood Vessels. 1991. PMID: 2001465 Review.
-
Endothelial-smooth muscle cell interactions in the regulation of vascular tone in skeletal muscle.Microcirculation. 2016 Nov;23(8):626-630. doi: 10.1111/micc.12322. Microcirculation. 2016. PMID: 27653241 Review.
Cited by
-
Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels.Skelet Muscle. 2019 Nov 6;9(1):27. doi: 10.1186/s13395-019-0213-2. Skelet Muscle. 2019. PMID: 31694693 Free PMC article.
-
Potassium Channels in the Uterine Vasculature: Role in Healthy and Complicated Pregnancies.Int J Mol Sci. 2022 Aug 21;23(16):9446. doi: 10.3390/ijms23169446. Int J Mol Sci. 2022. PMID: 36012712 Free PMC article. Review.
-
Impact of aging on vascular ion channels: perspectives and knowledge gaps across major organ systems.Am J Physiol Heart Circ Physiol. 2023 Nov 1;325(5):H1012-H1038. doi: 10.1152/ajpheart.00288.2023. Epub 2023 Aug 25. Am J Physiol Heart Circ Physiol. 2023. PMID: 37624095 Free PMC article. Review.
-
Role of perivascular nerve and sensory neurotransmitter dysfunction in inflammatory bowel disease.Am J Physiol Heart Circ Physiol. 2021 May 1;320(5):H1887-H1902. doi: 10.1152/ajpheart.00037.2021. Epub 2021 Mar 12. Am J Physiol Heart Circ Physiol. 2021. PMID: 33710922 Free PMC article.
-
Myofibre injury induces capillary disruption and regeneration of disorganized microvascular networks.J Physiol. 2022 Jan;600(1):41-60. doi: 10.1113/JP282292. Epub 2021 Dec 8. J Physiol. 2022. PMID: 34761825 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

