Primary cilia disruption differentially affects the infiltrating and resident macrophage compartment in the liver
- PMID: 29543508
- PMCID: PMC6048441
- DOI: 10.1152/ajpgi.00381.2017
Primary cilia disruption differentially affects the infiltrating and resident macrophage compartment in the liver
Abstract
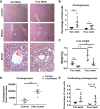
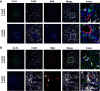
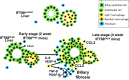
Hepatorenal fibrocystic disease (HRFCD) is characterized by cysts in the kidney and liver with associated fibrosis and is the result of defects in proteins required for cilia function or assembly. Previous reports indicate that macrophages, mainly M2-like macrophages, contribute to HRFCD, although the origin of these cells (yolk sac-derived resident macrophages vs. bone marrow-derived infiltrating macrophages) and their contribution to the observed phenotypes are unknown. We utilize a congenital model of cilia dysfunction (IFT88Orpk) to study the importance of macrophages in HRFCD. Our data show a rapid expansion of the bile duct region and development of fibrosis between 2 and 4 wk of age. Immunofluorescence microscopy analysis reveals an accumulation of F4/80+ macrophages in regions exhibiting biliary hyperplasia in IFT88Orpk mice. Flow cytometry data show that cilia dysfunction leads to an accumulation of infiltrating macrophages (CD11bhi, F4/80lo) and a reduction of resident macrophage (CD11blo, F4/80hi) number. A majority of the infiltrating macrophages are Ly6chi profibrogenic macrophages. Along with the accumulation of immune cells, expression of proinflammatory and profibrotic transcripts, including TGF-β, TNF-α, IL-1β, and chemokine (C-C) motif ligand 2, is increased. Quantitative RT-PCR analysis of flow-sorted cells shows enhanced expression of CCL2 in cholangiocytes and enhanced expression of VEGF-A and IL-6 in Ly6chi macrophages. Genetic inhibition of Ly6chi macrophage accumulation in IFT88Orpk FVB CCR2-/- mice reduced biliary fibrosis but did not affect epithelial expansion. Collectively, these studies suggest that biliary epithelium with defects in primary cilia preferentially recruits Ly6chi infiltrating macrophages, which promote fibrotic progression in HRFCD pathogenesis. NEW & NOTEWORTHY These studies are the first to address the contribution of the infiltrating and resident macrophage niche during progression of hepatorenal fibrocystic disease (HRFCD). We show that the number of infiltrating macrophages is significantly upregulated in HRFCD mouse models. Finally, we show that prevention of Ly6chi infiltrating macrophage accumulation significantly reduces biliary fibrosis, but not biliary hyperplasia, suggesting that this population may be responsible for the fibrotic progression of the disease in HRFCD patients.
Keywords: cholangiocyte; hepatorenal fibrocystic disease; liver; macrophage.
Figures






References
-
- Chen LP, Qian YY, Li ZL, Bai HW, Cai M, Shi BY. [Role of IL-6/STAT3 in rat cholangiocyte proliferation induced by lipopolysaccharide]. Zhonghua Gan Zang Bing Za Zhi 17: 374–377, 2009. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

