Development and evaluation of a novel high-throughput image-based fluorescent neutralization test for detection of Zika virus infection
- PMID: 29543803
- PMCID: PMC5871014
- DOI: 10.1371/journal.pntd.0006342
Development and evaluation of a novel high-throughput image-based fluorescent neutralization test for detection of Zika virus infection
Abstract
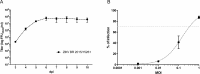
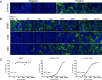
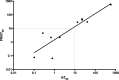
Zika virus (ZIKV) is an emerging arbovirus belonging to the genus flavivirus that comprises other important public health viruses, such as dengue (DENV) and yellow fever (YFV). In general, ZIKV infection is a self-limiting disease, however cases of Guillain-Barré syndrome and congenital brain abnormalities in newborn infants have been reported. Diagnosing ZIKV infection remains a challenge, as viral RNA detection is only applicable until a few days after the onset of symptoms. After that, serological tests must be applied, and, as expected, high cross-reactivity between ZIKV and other flavivirus serology is observed. Plaque reduction neutralization test (PRNT) is indicated to confirm positive samples for being more specific, however it is laborious intensive and time consuming, representing a major bottleneck for patient diagnosis. To overcome this limitation, we developed a high-throughput image-based fluorescent neutralization test for ZIKV infection by serological detection. Using 226 human specimens, we showed that the new test presented higher throughput than traditional PRNT, maintaining the correlation between results. Furthermore, when tested with dengue virus samples, it showed 50.53% less cross reactivity than MAC-ELISA. This fluorescent neutralization test could be used for clinical diagnosis confirmation of ZIKV infection, as well as for vaccine clinical trials and seroprevalence studies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lindenbach BD, Thiel H-J, Rice CM (2007) Flaviviridae: The Viruses and Their Replication In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott- Raven; pp. 1101–1152.
-
- Dick G, Kitchen S, Haddow A (1952) Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 46: 509–520. doi: 10.1016/0035-9203(52)90042-4 - DOI - PubMed
-
- Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, et al. (2009) Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360: 2536–2543. doi: 10.1056/NEJMoa0805715 - DOI - PubMed
-
- Hancock WT, Marfel M, Bel M (2014) Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis 20: 1960 doi: 10.3201/eid2011.141380 - DOI - PMC - PubMed
-
- Zanluca C, Duarte dos Santos CN (2016) Zika virus: an overview. Microbes Infect 18: 295–301. Available: doi: 10.1016/j.micinf.2016.03.003 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

