The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: A randomized, controlled trial
- PMID: 29543887
- PMCID: PMC5854344
- DOI: 10.1371/journal.pone.0193846
The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: A randomized, controlled trial
Abstract
Introduction: The clinical utility of denosumab for the treatment of glucocorticoid-induced osteoporosis (GIOP) has yet to be established. This study aimed to compare the effects of denosumab on bone mineral density (BMD) and bone turnover markers to those of alendronate in patients with GIOP.
Methods: A prospective, single-center study of 32 patients (18 men; median age, 66.0 years) with glomerular disease receiving prednisolone (PSL) who were diagnosed as having GIOP and had not received bisphosphonates before was conducted. Participants were randomized to either alendronate (35 mg orally once a week) or denosumab (60 mg subcutaneously once every 6 months), and all subjects received calcitriol. The primary endpoint was the percent change in lumbar spine (LS) BMD at 12 months of treatment.
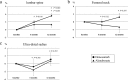
Results: The demographic and clinical characteristics at baseline were not significantly different between the groups. Denosumab treatment markedly decreased serum levels of t-PINP, BAP, and TRACP-5b at 12 months compared to baseline (-57.4%, p<0.001; -30.9%, p<0.01; -57.7%, p<0.001, respectively). After 12 months of alendronate treatment, serum levels of t-PINP, BAP, and TRACP-5b were also significantly decreased compared to pretreatment (-38.9%, p<0.01; -16.3%, p<0.05; -43.5%, p<0.01, respectively). However, no significant differences in the changes of bone turnover markers were found between the two groups. As for the effects on BMD, denosumab treatment markedly increased LS BMD from 6 months compared to baseline, whereas no significant difference compared to pretreatment was found in the alendronate group during the study period. In the comparison of the two groups, a large increase of LS BMD was found in the denosumab treatment group compared to the alendronate treatment group at 12 months (p<0.05).
Conclusions: In patients with GIOP, denosumab treatment markedly suppressed bone turnover, which led to a significantly greater increase in LS BMD than with alendronate treatment. These results suggest that denosumab is a therapeutic option for the treatment of GIOP.
Conflict of interest statement
Figures

References
-
- LoCascio V, Bonucci E, Imbimbo B, Ballanti P, Adami S, Milani S, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8(1):39–51. - PubMed
-
- van Staa TP, Leufkens HGM, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002. October;13(10):777–87. doi: 10.1007/s001980200108 - DOI - PubMed
-
- Weinstein RS. Glucocorticoid-Induced Bone Disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926 - DOI - PubMed
-
- Rizzoli R, Biver E. Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol. 2015. February;11(2):98–109. doi: 10.1038/nrrheum.2014.188 - DOI - PubMed
-
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003. April;111(8):1221–30. doi: 10.1172/JCI17215 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

