Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial
- PMID: 29543938
- PMCID: PMC5885246
- DOI: 10.1001/jamaoncol.2017.5847
Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial
Abstract
Importance: Patients with hepatocellular carcinoma showing macroscopic vascular invasion have a poor prognosis. Sorafenib is the sole treatment option for these patients, with unsatisfactory response and survival benefit. Combined treatment with transarterial chemoembolization (TACE) plus external beam radiotherapy (RT) has shown promising results for these patients in observational studies.
Objective: To evaluate the efficacy and safety of TACE plus RT compared with sorafenib for patients with hepatocellular carcinoma and macroscopic vascular invasion.
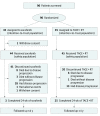
Design, setting, and participants: In this randomized, open-label clinical trial conducted at an academic tertiary care center between July 1, 2013, and October 31, 2016, 90 treatment-naive patients with liver-confined hepatocellular carcinoma showing macroscopic vascular invasion were randomly assigned to receive sorafenib (400 mg twice daily; 45 participants [the sorafenib group]) or TACE (every 6 weeks) plus RT (within 3 weeks after the first TACE, maximum 45 Gy with the fraction size of 2.5 to 3 Gy; 45 participants [the TACE-RT group]).
Main outcomes and measures: The primary end point was the 12-week progression-free survival rate by intention-to-treat analysis. Radiologic response was assessed by independent review according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1). Treatment crossover was permitted after confirming disease progression.
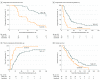
Results: Of the 90 patients (median age, 55 years; range, 33-82 years), 77 were men and 13 were women. All patients had portal vein invasion of hepatocellular carcinoma and Child-Pugh class A liver function. The median maximal tumor diameter was 9.7 cm. Most patients (71 [78.9%]) had multiple lesions. At week 12, the progression-free survival rate was significantly higher in the TACE-RT group than the sorafenib group (86.7% vs 34.3%; P < .001). The TACE-RT group showed a significantly higher radiologic response rate than the sorafenib group at 24 weeks (15 [33.3%] vs 1 [2.2%]; P < .001), a significantly longer median time to progression (31.0 vs 11.7 weeks; P < .001), and significantly longer overall survival (55.0 vs 43.0 weeks; P = .04). Curative surgical resection was conducted for 5 patients (11.1%) in the TACE-RT group owing to downstaging. No patients in the TACE-RT group discontinued treatment owing to hepatic decompensation.
Conclusions and relevance: For patients with advanced hepatocellular carcinoma showing macroscopic vascular invasion, first-line treatment with TACE plus RT was well tolerated and provided an improved progression-free survival, objective response rate, time to progression, and overall survival compared with sorafenib treatment.
Trial registration: clinicaltrials.gov Identifier: NCT01901692.
Conflict of interest statement
Figures
Comment in
-
Hepatocellular Carcinoma With Portal Venous Invasion: Radiating New Hope?JAMA Oncol. 2018 May 1;4(5):669-670. doi: 10.1001/jamaoncol.2018.0007. JAMA Oncol. 2018. PMID: 29543953 No abstract available.
-
Locoregional versus systemic therapy - robust positive data remain elusive.Nat Rev Clin Oncol. 2018 Sep;15(9):537-538. doi: 10.1038/s41571-018-0047-6. Nat Rev Clin Oncol. 2018. PMID: 29858583 No abstract available.
-
Gastrointestinal Cancers: Management of Rectal, Hepatocellular, Pancreatic, and Esophageal Cancers.Int J Radiat Oncol Biol Phys. 2019 May 1;104(1):1-9. doi: 10.1016/j.ijrobp.2018.12.052. Int J Radiat Oncol Biol Phys. 2019. PMID: 30967220 No abstract available.
References
-
- International Agency for Research on Cancer, World Health Organization Estimated number of deaths, both sexes, worldwide (top 10 cancer sites) in 2012. http://gco.iarc.fr/today/online-analysis-multi-bars?mode=cancer&mode_pop.... Accessed September 1, 2017.
-
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943. - PubMed
-
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Allen C, Barber RM, et al. . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease study. JAMA Oncol. 2017;3(4):524-548. - PMC - PubMed
-
- Kim BH, Lim YS, Kim EY, et al. . Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus–endemic population [published online June 14, 2017]. J Gastroenterol Hepatol. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

