Macrolide antibiotics for bronchiectasis
- PMID: 29543980
- PMCID: PMC6494352
- DOI: 10.1002/14651858.CD012406.pub2
Macrolide antibiotics for bronchiectasis
Abstract
Background: Bronchiectasis is a chronic respiratory disease characterised by abnormal and irreversible dilatation and distortion of the smaller airways. Bacterial colonisation of the damaged airways leads to chronic cough and sputum production, often with breathlessness and further structural damage to the airways. Long-term macrolide antibiotic therapy may suppress bacterial infection and reduce inflammation, leading to fewer exacerbations, fewer symptoms, improved lung function, and improved quality of life. Further evidence is required on the efficacy of macrolides in terms of specific bacterial eradication and the extent of antibiotic resistance.
Objectives: To determine the impact of macrolide antibiotics in the treatment of adults and children with bronchiectasis.
Search methods: We identified trials from the Cochrane Airways Trials Register, which contains studies identified through multiple electronic searches and handsearches of other sources. We also searched trial registries and reference lists of primary studies. We conducted all searches on 18 January 2018.
Selection criteria: We included randomised controlled trials (RCTs) of at least four weeks' duration that compared macrolide antibiotics with placebo or no intervention for the long-term management of stable bronchiectasis in adults or children with a diagnosis of bronchiectasis by bronchography, plain film chest radiograph, or high-resolution computed tomography. We excluded studies in which participants had received continuous or high-dose antibiotics immediately before enrolment or before a diagnosis of cystic fibrosis, sarcoidosis, or allergic bronchopulmonary aspergillosis. Our primary outcomes were exacerbation, hospitalisation, and serious adverse events.
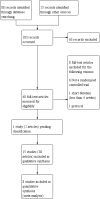
Data collection and analysis: Two review authors independently screened the titles and abstracts of 103 records. We independently screened the full text of 40 study reports and included 15 trials from 30 reports. Two review authors independently extracted outcome data and assessed risk of bias for each study. We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We used standard methodological procedures as expected by Cochrane.
Main results: We included 14 parallel-group RCTs and one cross-over RCT with interventions lasting from 8 weeks to 24 months. Of 11 adult studies with 690 participants, six used azithromycin, four roxithromycin, and one erythromycin. Four studies with 190 children used either azithromycin, clarithromycin, erythromycin, or roxithromycin.We included nine adult studies in our comparison between macrolides and placebo and two in our comparison with no intervention. We included one study with children in our comparison between macrolides and placebo and one in our comparison with no intervention.In adults, macrolides reduced exacerbation frequency to a greater extent than placebo (OR 0.34, 95% confidence interval (CI) 0.22 to 0.54; 341 participants; three studies; I2 = 65%; moderate-quality evidence). This translates to a number needed to treat for an additional beneficial outcome of 4 (95% CI 3 to 8). Data show no differences in exacerbation frequency between use of macrolides (OR 0.31, 95% CI 0.08 to 1.15; 43 participants; one study; moderate-quality evidence) and no intervention. Macrolides were also associated with a significantly better quality of life compared with placebo (MD -8.90, 95% CI -13.13 to -4.67; 68 participants; one study; moderate-quality evidence). We found no evidence of a reduction in hospitalisations (OR 0.56, 95% CI 0.19 to 1.62; 151 participants; two studies; I2 = 0%; low-quality evidence), in the number of participants with serious adverse events, including pneumonia, respiratory and non-respiratory infections, haemoptysis, and gastroenteritis (OR 0.49, 95% CI 0.20 to 1.23; 326 participants; three studies; I2 = 0%; low-quality evidence), or in the number experiencing adverse events (OR 0.83, 95% CI 0.51 to 1.35; 435 participants; five studies; I2 = 28%) in adults with macrolides compared with placebo.In children, there were no differences in exacerbation frequency (OR 0.40, 95% CI 0.11 to 1.41; 89 children; one study; low-quality evidence); hospitalisations (OR 0.28, 95% CI 0.07 to 1.11; 89 children; one study; low-quality evidence), serious adverse events, defined within the study as exacerbations of bronchiectasis or investigations related to bronchiectasis (OR 0.43, 95% CI 0.17 to 1.05; 89 children; one study; low-quality evidence), or adverse events (OR 0.78, 95% CI 0.33 to 1.83; 89 children; one study), in those receiving macrolides compared to placebo. The same study reported an increase in macrolide-resistant bacteria (OR 7.13, 95% CI 2.13 to 23.79; 89 children; one study), an increase in resistance to Streptococcus pneumoniae (OR 13.20, 95% CI 1.61 to 108.19; 89 children; one study), and an increase in resistance to Staphylococcus aureus (OR 4.16, 95% CI 1.06 to 16.32; 89 children; one study) with macrolides compared with placebo. Quality of life was not reported in the studies with children.
Authors' conclusions: Long-term macrolide therapy may reduce the frequency of exacerbations and improve quality of life, although supporting evidence is derived mainly from studies of azithromycin, rather than other macrolides, and predominantly among adults rather than children. However, macrolides should be used with caution, as limited data indicate an associated increase in microbial resistance. Macrolides are associated with increased risk of cardiovascular death and other serious adverse events in other populations, and available data cannot exclude a similar risk among patients with bronchiectasis.
Conflict of interest statement
Sally Spencer, Carol Kelly, and Nicola Relph were named co‐investigators on a study funded by Edge Hill University to develop a series of reviews on bronchiectasis. Lambert Felix was supported by that funding. No funding was received by any other review authors for participation in this systematic review.
David Evans provides freelance writing services to medical communication agencies. Steve Milan: none known.
Iain Crossingham received travel and training expenses from Hamilton Medical that are not connected to the topic of this review.
James D Chalmers declares grant support from Pfizer, AstraZeneca, and GlaxoSmithKline. In addition, he is part of an innovative medicines initiative consortium that includes Novartis and Basilea. He has participated in advisory boards for Bayer HealthCare, Chiesi, and Raptor Pharmaceuticals. He has received fees for speaking from Napp, AstraZeneca, BI, and Pfizer. None of these conflicts of interest are related to the work involved in this review, and these conflicts are unrelated to the topic of this review.
Figures
Update of
References
References to studies included in this review
Altenburg 2013 {published data only}
-
- Altenburg J, Wilms E, Boersma W. The relationship between serum‐ and sputum levels of azithromycin and clinical endpoints in bronchiectasis patients in bronchiectasis patients using maintenance treatment. Pneumologie. 2016; Vol. 70:A3. [DOI: 10.1055/s-0036-1592227] - DOI
-
- Altenburg J, Wolf R, Go S, Rijn P, Boersma W, Werf T. Changes of computed tomography features of bronchiectasis during one year of azithromycin treatment. European Respiratory Journal. 2016; Vol. 48:OA278. [DOI: 10.1183/13993003.congress-2016.OA278] - DOI
-
- Altenburg J, Graaf C, Werf T, Boersma W. Long term azithromycin treatment: A randomised placebo‐controlled trial in non‐CF bronchiectasis; results from the BAT trial. European Respiratory Journal. 2011; Vol. 38:1924.
-
- Altenburg J, Graaf CS, Steinstra Y, Sloos JH, Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309(12):1251‐9. - PubMed
-
- Boersma WG, Altenburg J, Werf TS. Evaluation of symptoms score and QoL in azithromycin maintenance treatment: results of a RCT trial in patients with bronchiectasis. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185:A3658.
Asintam 2012 {published data only}
-
- Asintam P, Kiranantawat N, Juthong S. Can roxithromycin improve quality of life in bronchiectatic patients?. European Respiratory Journal. 2012; Vol. 40:P2168.
Cymbala 2005 {published data only}
-
- Cymbala AA, Edmonds LC, Bauer MA, Jederlinic PJ, May JJ, Victory JM, et al. The disease‐modifying effects of twice‐weekly oral azithromycin in patients with bronchiectasis. Treatments in Respiratory Medicine 2005;4(2):117‐22. - PubMed
Diego 2013 {published data only}
-
- Diego AD, Milara J, Martinez‐Moragón E, Palop M, León M, Cortijo J. Effects of long‐term azithromycin therapy on airway oxidative stress markers in non‐cystic fibrosis bronchiectasis. Respirology 2013;18(7):1056‐62. - PubMed
Juthong 2011 {published data only}
-
- Juthong S, Eiamsaard S. The effects of roxithromycin as anti‐inflammatory agent on clinical outcomes in patient with bronchiectasis: a double blinded randomized controlled study. European Respiratory Journal. 2011; Vol. 38(Suppl 55):455s.
Koh 1997 {published data only}
-
- Koh YY, Lee MH, Sun YH, Sung KW, Chae JH. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double‐blind, placebo‐controlled study. European Respiratory Journal 1997;10(5):994‐9. [PUBMED: 9163637] - PubMed
Liu 2012 {published data only}
-
- Liu JF, Zhong XN, He ZY, Zhong DJ, Bai J, Zhang JQ, et al. [Impact of treatment with low dose roxithromycin on stable bronchiectasis]. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua jiehe he huxi zazhi = Chinese Journal of Tuberculosis and Respiratory Diseases 2012;35(11):824‐7. [PUBMED: 23290037] - PubMed
Liu 2014 {published data only}
Lourdesamy 2014 {published data only}
-
- Lourdesamy Anthony AI, Muthukumaru U. Efficacy of azithromycin in the treatment of bronchiectasis. Respirology (Carlton, Vic.) 2014;19(8):1178‐82. [PUBMED: 25183304] - PubMed
Masekela 2013 {published data only}
-
- Masekela R, Anderson R, Gongxeka H, Steel HC, Green RJ. Lack of efficacy of erythromycin in children with human immunodeficiency virus‐related bronchiectasis – a randomised controlled trial. Paediatric Respiratory Reviews. 2013; Vol. 14, issue 2:S82/A009‐12. [DOI: ]
-
- Masekela R, Anderson R, Gongxeka H, Steel HC, et al. Lack of efficacy of an immunomodulatory macrolide in childhood HIV‐related bronchiectasis: a randomised, placebo‐controlled trial. Journal of Antivirals and Antiretrovirals 2013;5:44‐9.
Sadigov 2013 {published data only}
-
- Sadigov AS, Mammadov GT. Azythromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis: how we can improve the clinical features of severe disease?. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187:A3512.
Serisier 2013 {published data only}
-
- Burr L, Rogers G, Taylor S, McGuckin M, Serisier D. Sub inhibitory erythromycin reduces the expression of key P. Aeruginosa virulence determinants in non‐CF bronchiectasis subjects. Respirology. 2015:29.
-
- Burr LD, Rogers GB, Chen AC, Hamilton BR, Pool GF, Taylor SL, et al. Macrolide treatment inhibits Pseudomonas aeruginosa quorum sensing in non‐cystic fibrosis bronchiectasis. An analysis from the bronchiectasis and low‐dose erythromycin study trial. Annals of the American Thoracic Society 2016;13(10):1697‐703. [PUBMED: 27464029] - PubMed
-
- Chen AC, Martin MM, Burr L, Hasnain SZ, Lourie R, Bowler SD, et al. Clinical benefits of long‐term, low‐dose erythromycin in bronchiectasis are not due to anti‐inflammatory effects. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187:A5970.
-
- Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. Corrections. The effect of long‐term macrolide treatment on respiratory microbiota composition in non‐cystic fibrosis bronchiectasis: an analysis from the randomised, double‐blind, placebo‐controlled BLESS trial. Lancet Respiratory Medicine 2015; Vol. 3, issue 4:e15. [PUBMED: 25890660] - PubMed
-
- Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long‐term macrolide treatment on respiratory microbiota composition in non‐cystic fibrosis bronchiectasis: an analysis from the randomised, double‐blind, placebo‐controlled BLESS trial. Lancet Respiratory Medicine 2014;2(12):988‐96. [PUBMED: 25458200] - PubMed
Valery 2013 {published data only}
-
- Hare KM, Grimwood K, Chang AB, Chatfield MD, Valery PC, Leach AJ, et al. Nasopharyngeal carriage and macrolide resistance in indigenous children with bronchiectasis randomized to long‐term azithromycin or placebo. European Journal of Clinical Microbiology and Infectious Diseases 2015;34(11):2275‐85. [PUBMED: 26363637] - PubMed
-
- Singleton R, Morris P, Leach A, Roseby R, White A, Valery P, et al. [Multicentre bronchiectasis study: an international observational and interventional study of bronchiectasis in indigenous children]. Respirology. 2008; Vol. 13(Suppl 2):A19.
-
- Singleton R, Morris P, Leach A, Roseby R, White A, Valery PC, et al. BIS ‐ Multi‐centre bronchiectasis study: a collaborative and international study of bronchiectasis in indigenous children. Respirology 2007; Vol. 12, issue 4:A‐192.
-
- Valery PC, Morris PS, Byrnes CA, Grimwood K, Torzillo PJ, Bauert PA, et al. Long‐term azithromycin for indigenous children with non‐cystic‐fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double‐blind, randomised controlled trial. Lancet Respiratory Medicine 2013;1(8):610‐20. [PUBMED: 24461664] - PubMed
Wong 2012 {published data only}
-
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis (EMBRACE): a randomised, double‐blind, placebo‐controlled trial. Lancet 2012;380(9842):660‐7. [PUBMED: 22901887] - PubMed
-
- Wong CA, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin decreases exacerbations in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine. 2012a; Vol. 185:A3657.
Yalcin 2006 {published data only}
-
- Yalcin E, Kiper N, Ozcelik U, Dogru D, Firat P, Sahin A, et al. Effects of clarithromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. Journal of Clinical Pharmacy and Therapeutics 2006;31(1):49‐55. [PUBMED: 16476120] - PubMed
References to studies excluded from this review
Chang 2013 {published data only}
Kudo 1988 {published data only}
-
- Kudo K, Komase Y, Kowada A, Kabe J. Preclinical and clinical studies on TE‐031 (A‐56268) in treatment of bacterial respiratory tract infections. Chemotherapy 1988;36(Suppl 3):617‐22.
Min 1988 {published data only}
-
- Min KY, Kuriyama T, Fukuday Y. Clinical evaluation of TE‐031 in the chronic respiratory infections [Japanese]. Japanese Pharmacology and Therapeutics 1988;16(7):3027‐39.
Ming 2005 {published data only}
-
- Ming O, Yong L, Zhang W. Efficacy of macrolide and theophylline in the management of bronchiectasis. Respirology 2005;10:A168.
Rikitomi 1988 {published data only}
-
- Rikitomi N, Shishido H, Nagatake T, Mbaki U, Matsumoto K. Preclinical and clinical studies on TE‐031 (A‐56268) in treatment of bacterial respiratory tract infections. Chemotherapy 1988;36(Suppl 3):715‐28.
Saito 1988 {published data only}
-
- Saito A, Shimada J, Ohmori M, Shiba K, Yamaji K, Hojo T, et al. Clinical studies of TE‐031 (A‐56268). Chemotherapy 1988;38:576‐85.
Tagaya 2002 {published data only}
-
- Tagaya E, Tamaoki J, Kondo M, Nagai A. Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest 2002;122(1):213‐8. [PUBMED: 12114361] - PubMed
Unoura 1986 {published data only}
-
- Unoura T, Masuda M, Takeuchi K, Itoh T, Tamura M, Yuki T, et al. Clinical study on TE‐031(A‐56268) against respiratory infections. Physicians' Therapy Manual 1988;36:544‐8.
References to studies awaiting assessment
Tsang 1999 {published data only}
-
- Tsang KW, Ho PI, Chan KN, Ip MS, Lam WK, Ho CS, et al. A pilot study of low‐dose erythromycin in bronchiectasis. European Respiratory Journal 1999;13(2):361‐4. [PUBMED: 10065682] - PubMed
-
- Tsang KW, Ho PL, Chan KN, Ip M, Lam WK, Lam B, et al. Erythromycin (EM) reduces sputum volume and improves lung functions in bronchiectasis. American Thoracic Society International Conference; 1998 April 24‐29; Chicago. 1998; Vol. A58:A174.
Additional references
Albert 2011
Aliberti 2016
-
- Aliberti S, Masefield S, Polverino E, Soyza A, Loebinger MR, Menendez R, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. European Respiratory Journal 2016;48(3):632‐47. - PubMed
Amsden 2005
-
- Amsden GW. Anti‐inflammatory effects of macrolides ‐ an underappreciated benefit in the treatment of community‐acquired respiratory tract infections and chronic inflammatory pulmonary conditions?. Journal of Antimicrobial Chemotherapy 2005;55(1):10‐21. - PubMed
Brodt 2014
-
- Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non‐cystic fibrosis bronchiectasis: a systematic review. European Respiratory Journal 2014;44(2):382‐93. - PubMed
Chalmers 2012
-
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. - PubMed
Chalmers 2014
Chang 2002
-
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [PUBMED: 12175325] - PubMed
Chang 2010
-
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes P, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand: a position statement from the Thoracic Society of Australia and New Zealand and the Australian Lung Foundation. Medical Journal of Australia 2010;193(6):356‐65. - PubMed
European Lung White Book 2013
-
- Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B, editor(s). European Lung White Book: Respiratory Health and Disease in Europe. European Respiratory Society, 2013. https://www.erswhitebook.org/ (accessed before 11 January 2018). - PubMed
Finch 2015
-
- Finch S, McDonnell MJ, Abo‐Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Annals of the American Thoracic Society 2015;12(11):1602‐11. - PubMed
Gao 2014
Goeminne 2016
-
- Goeminne PC, Soyza A. Bronchiectasis: how to be an orphan with many parents?. European Respiratory Journal 2016;47(1):10‐3. - PubMed
GRADEproGDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 14 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
Hansen 2015
Haworth 2014
-
- Haworth CS, Bilton D, Elborn JS. Long term macrolide maintenance therapy in non‐CF bronchiectasis: evidence and questions. Respiratory Medicine 2014;108(1):1397‐408. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hnin 2015
Joish 2013
-
- Joish VN, Spilsbury‐Cantalupo M, Operschall E, Luong B, Boklage S. Economic burden of non‐cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Applied Health Economics and Health Policy 2013;11(3):299‐304. - PubMed
Kapur 2012
-
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141(4):1018‐24. [PUBMED: 21885727] - PubMed
Kohler 2010
Kwak 2010
-
- Kwak HJ, Moon JY, Choi YW, Kim TH, Sohn JW, Yoon HJ, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku Journal of Experimental Medicine 2010;222(4):237‐42. - PubMed
Leclercq 2002
-
- Leclercq R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clinical Infectious Diseases 2002;34(4):482‐92. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Martínez‐García 2007
-
- Martínez‐García MA, Soler‐Cataluña JJ, Perpiñá‐Tordera M, Román‐Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. - PubMed
Moher 2009
Pasteur 2010
-
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis (non‐CF) Guideline Group. British Thoracic Society Guidelines for Non‐CF Bronchiectasis. Thorax 2010;65(Suppl 1):i1‐58. - PubMed
Quint 2016
-
- Quint JK, Millett ERC, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. European Respiratory Journal 2016;47(1):186‐93. [DOI: 10.1183/13993003.01033-2015] - DOI - PMC - PubMed
Ray 2012
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringshausen 2015
-
- Ringshausen FC, Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population‐based estimation of disease prevalence. European Respiratory Journal 2015;46(6):1805‐7. [PUBMED: 26293498] - PubMed
Roberts 2010
-
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104:981‐5. - PubMed
Saiman 2003
-
- Saiman L, Marshall BC, Mayer‐Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290(13):1749‐56. - PubMed
Seitz 2010
Seitz 2012
Serisier 2013a
-
- Serisier DJ. Risk of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respiratory Medicine 2013;1(3):262‐74. - PubMed
Twiss 2005
Welsh 2015
Weycker 2005
-
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205‐9.
Wurzel 2011
Zarogoulidis 2012
-
- Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides from in vitro anti‐inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. European Journal of Clinical Pharmacology 2012;68(5):479‐503. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

